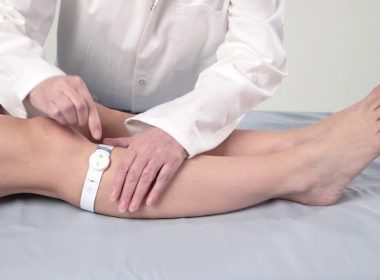
DARESBURY, England, March 25, 2022 /PRNewswire/ –Sky Medical Technology Ltd (Sky) has today announced it has achieved further U.S. Food and Drug Administration (FDA) 510(k) clearance to market the new (W3) geko™ device variant, for increasing microcirculatory blood flow in lower limb soft tissue of patients with venous insufficiency and/or ischemia. This latest […]