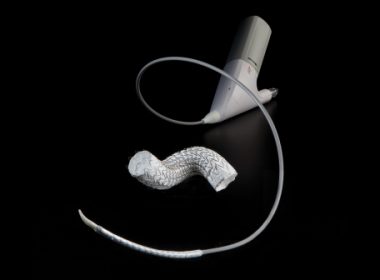
FLAGSTAFF, Ariz., Nov. 30, 2020 /PRNewswire/ — W. L. Gore & Associates, Inc. (Gore), a global leader in providing solutions for cardiovascular disease, today announced encouraging clinical results from its early feasibility study evaluating the safety and performance of its pulmonary valved conduit, an investigational device. Six-month data for 16 patients enrolled […]
Tag: Gore
Gore Innovation Center Announces Collaboration With Robotics Startup Moray Medical
Startups join Gore Innovation Center to bring new technologies to market. SANTA CLARA, Calif., Feb. 26, 2020 /PRNewswire/ — The Gore Innovation Center today announced a collaboration with Moray Medical, a 2-year-old startup developing precision automated delivery systems for endovascular procedures. The Gore Innovation Center collaboration with Moray Medical is aimed at fundamentally improving the […]
Gore Named a Fortune 100 Best Company to Work For®
NEWARK, Del., Feb. 18, 2020 /PRNewswire/ — W. L. Gore & Associates — a materials science company known for its innovative products and work environment — earned a spot on the 2020 Fortune 100 Best Companies to Work For® list, announced today. This marks Gore’s 21st appearance since the rankings debuted in 1998. […]
First U.S. Patient Receives GORE® TAG® Conformable Thoracic Stent Graft with ACTIVE CONTROL
FLAGSTAFF, Ariz.–(BUSINESS WIRE)–W. L. Gore & Associates, Inc. (Gore) announces the first U.S. implant of the GORE® TAG® Conformable Thoracic Stent Graft with ACTIVE CONTROL System. The successful procedure was performed by William Jordan, M.D., chief of the Division of Vascular Surgery and Bradley Leshnower, M.D., cardiothoracic surgeon, at the Emory […]
GORE® CARDIOFORM ASD Occluder Receives FDA Approval for the Treatment of Atrial Septal Defects
FLAGSTAFF, Ariz., June 4, 2019 /PRNewswire/ — W. L. Gore & Associates (Gore) announced the U.S. Food and Drug Administration’s (FDA’s) premarket approval (PMA) of the GORE® CARDIOFORM ASD Occluder for the percutaneous closure of ostium secundum atrial septal defects (ASDs). The FDA approval was supported by data collected from the pivotal stage of the Gore […]
Gore Receives FDA Approval for the GORE® TAG® Conformable Thoracic Stent Graft with ACTIVE CONTROL
FLAGSTAFF, Ariz.–(BUSINESS WIRE)–W. L. Gore & Associates, Inc. (Gore) today announced that the U.S. Food and Drug Administration (FDA) has granted regulatory approval for commercial distribution for the GORE® TAG® Conformable Thoracic Stent Graft with ACTIVE CONTROL System, a unique Thoracic Endovascular Aortic Repair (TEVAR) solution combining new levels of control […]
GORE® TIGRIS® Vascular Stent Demonstrates High Patency Rates at 12 Months in Treating SFA and PPA Occlusive Lesions in Challenging Patients
FLAGSTAFF, Ariz.–(BUSINESS WIRE)–The GORE® TIGRIS® Vascular Stent, a flexible self-expanding stent to treat peripheral arterial disease (PAD) in the superficial femoral artery (SFA) and proximal popliteal artery (PPA) developed by W. L. Gore & Associates, Inc. (Gore), demonstrated that it is a safe and effective device that can be incorporated […]
Gore Introduces GORE® TAG® Conformable Thoracic Stent Graft with Reduced Profiles in Europe
FLAGSTAFF, Ariz.–(BUSINESS WIRE)–W. L. Gore & Associates, Inc. (Gore) today introduced reduced profiles for the most commonly used diameters of the GORE® TAG® Conformable Thoracic Stent Graft with ACTIVE CONTROL System. The reduced profile allows physicians to perform TEVAR in patients with smaller vessels where access is challenging and aortic anatomy is tortuous, […]
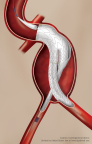
First Patient in Europe Receives Implant of GORE® EXCLUDER® Conformable AAA Endoprosthesis with ACTIVE CONTROL System and Enrolls in Post-Market Registry
FLAGSTAFF, Ariz.–(BUSINESS WIRE)–W. L. Gore & Associates, Inc. (Gore) today announced the first European patient implant of the GORE® EXCLUDER® Conformable AAA Endoprosthesis with ACTIVE CONTROL System. This next-generation endovascular aneurysm repair (EVAR) device is indicated to treat the broadest range of abdominal aortic aneurysms (AAA) in patients with challenging anatomies. […]
First Patient Enrolled in Investigational Study of the GORE EXCLUDER Conformable AAA Endoprosthesis with ACTIVE CONTROL System
FLAGSTAFF, Ariz.–(BUSINESS WIRE)– W. L. Gore & Associates, Inc.(Gore) today announced the first implant of the GORE® EXCLUDER® Conformable AAA Endoprosthesis in the United States. The successful procedure took place on December 19, 2017 at Maimonides Medical Center in New York by Robert Rhee, MD, Chief of Vascular and Endovascular Surgery, and National […]