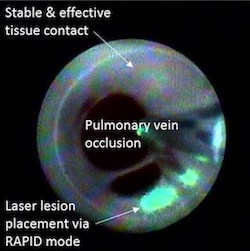
– Japan Lifeline will distribute CardioFocus’ innovative catheter ablation technology for pulmonary vein isolation, the gold standard treatment for atrial fibrillation – Regulatory approval comes as use of catheter ablation technologies for AFib is increasing in Japan at a substantial rate MARLBOROUGH, Mass., June 23, 2021 /PRNewswire/ — CardioFocus, Inc., a medical device […]
Tag: HeartLight X3
CardioFocus Announces Agreement with China Grand Pharmaceutical to Bring HeartLight X3 System to China
– China Grand Pharma will seek regulatory approvals and commercialize CardioFocus’ innovative catheter ablation technology for pulmonary vein isolation – Pulmonary vein isolation is the gold standard treatment for atrial fibrillation MARLBOROUGH, Mass., June 2, 2021 /PRNewswire/ — CardioFocus, Inc., a medical device company dedicated to advancing ablation treatments for atrial fibrillation (AFib), […]
CardioFocus Announces The Publication Of HeartLight X3 Pivotal Study Data
HeartLight® X3 System Has Significantly Shorter Procedure Times for Atrial Fibrillation Ablation MARLBOROUGH, Mass., Feb. 25, 2021 /PRNewswire/ — CardioFocus, Inc., a medical device company dedicated to advancing ablation treatment for atrial fibrillation (AFib), announced today that results from its HeartLight® X3 Pivotal Study were published in the premier journal Circulation: Arrhythmia and Electrophysiology (CircEP), which features […]
CardioFocus Announces European CE Mark Approval Of Breakthrough HeartLight® X3 System For The Treatment Of Atrial Fibrillation
MARLBOROUGH, Mass., March 25, 2019 /PRNewswire/ — CardioFocus, Inc., a medical device company dedicated to advancing ablation treatment for atrial fibrillation (AFib), today announced the European CE Mark approval of the HeartLight X3 Endoscopic Ablation System. The HeartLight X3 System is a third generation technology building upon the advanced features of the HeartLight Endoscopic […]
CardioFocus Completes Enrollment In HeartLight® X3 Trial
MARLBOROUGH, Mass., Nov. 29, 2018 /PRNewswire/ — CardioFocus, Inc., a medical device company dedicated to advancing ablation treatment for atrial fibrillation (AFib), today announced completed enrollment in a trial to evaluate its next-generation HeartLight® X3 Endoscopic Ablation System. A total of 60 patients have been treated in this pivotal confirmatory trial with a one-month […]