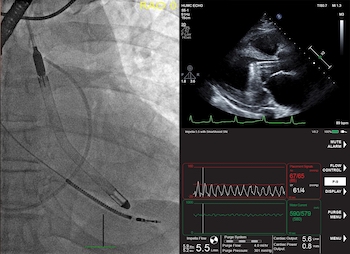
Danvers, Mass .- ( BUSINESS WIRE ) -Abiomed (NASDAQ: ABMD) is a large target of 356 patients treated with Impella 5.5 with SmartAssist at 16 facilities in the United States and Germany. A large study announced that the survival rate at withdrawal from support was 79%. The majority of surviving patients recovered autologous heart function and did not require additional mechanical […]
Tag: Impella 5.5
First 1,000 Patients Treated with Impella 5.5 with SmartAssist, a Heart Pump Designed for Surgeons
DANVERS, Mass.–(BUSINESS WIRE)–Abiomed (NASDAQ: ABMD) announces 1,000 patients have been treated with the Impella 5.5 with SmartAssist heart pump in the first year after the U.S. Food and Drug Administration (FDA) granted Impella 5.5 with SmartAssist its highest level of approval for safety and efficacy. In October 2019, the first U.S. patients were […]
Study Finds 84% Survival Rate in Patients in Cardiogenic Shock and Other Challenging Cardiac Conditions with the New Impella 5.5 with SmartAssist
DANVERS, Mass.–(BUSINESS WIRE)–The first published United States experience of patients who received Abiomed’s newest heart pump, Impella 5.5 with SmartAssist, finds 84% of the patients survived to explant with 76% native heart recovery. The study was published in the July edition of the American Society for Artificial Internal Organs (ASAIO) Journal. The study examined […]
Abiomed (ABMD) Reports European Approval (CE Marking) for Impella 5.5 & 1st Patient Treated at University Heart Center Hamburg
Abiomed, Inc. (NASDAQ: ABMD) announced today that the Impella 5.5™ heart pump received CE marking1 approval in Europe and the first patient was treated at University Heart Center in Hamburg, Germany. The Impella 5.5 heart pump further enhances Abiomed’s product portfolio and provides physicians a 30-day, ambulatory, wean-able, forward flow heart pump. […]