DANVERS, Mass.–(BUSINESS WIRE)–Abiomed (Nasdaq: ABMD) announces the United States Food and Drug Administration (FDA) has approved the version of Impella ECP that will be used in the Impella ECP Pivotal Trial, and the first two patients have been enrolled in the trial. Amir Kaki, MD, director of mechanical circulatory support at […]
Tag: Impella ECP
FDA Grants Breakthrough Device Designation to Impella ECP, the World’s Smallest Heart Pump
DANVERS, Mass.–(BUSINESS WIRE)–The United States Food and Drug Administration (FDA) has granted breakthrough device designation to Abiomed’s (NASDAQ: ABMD) Impella ECP expandable percutaneous heart pump. The designation means the FDA will prioritize Impella ECP’s regulatory review processes including design iterations, clinical study protocols and pre-market approval (PMA) application. Impella ECP is the smallest […]
First Patients Treated with the World’s Smallest Heart Pump, the 9Fr Impella ECP
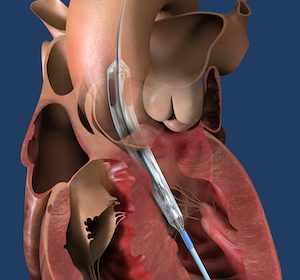
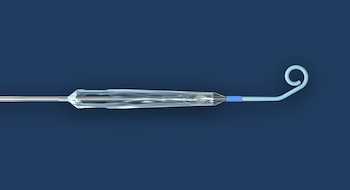
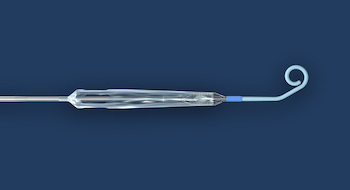
DANVERS, Mass.–(BUSINESS WIRE)–Abiomed (NASDAQ: ABMD) announces the first two patients have been treated with the Impella ECP expandable percutaneous heart pump. Impella ECP is the smallest heart pump in the world. It measures 9 French (Fr) (3 millimeters) in diameter upon insertion and removal from the body. While in the heart, […]
FDA Approves Abiomed’s First-in-Human Trial of Impella ECP, World’s Smallest Heart Pump
DANVERS, Mass.–(BUSINESS WIRE)–Abiomed (NASDAQ: ABMD) announces the United States Food and Drug Administration (FDA) has approved the company’s investigational device exemption application to start an early feasibility study with a first-in-human trial of the 9 French (Fr) Impella ECP™ heart pump. Impella ECP, which stands for expandable cardiac power, will be […]