Consistent evidence supports benefits of complete revascularization on LVEF, heart failure symptoms DANVERS, Mass.–(BUSINESS WIRE)–Abiomed (Nasdaq: ABMD) announces the results of the Restore EF study demonstrate Impella-supported high-risk percutaneous coronary intervention (PCI) leads to significant improvements in left ventricular ejection fraction (LVEF), angina symptoms and heart failure symptoms at follow-up. The study, […]
Tag: Impella
Two Large Studies Demonstrate Complete Revascularization with Impella Heart Pumps Improves Ejection Fraction and Long-Term Patient Outcomes
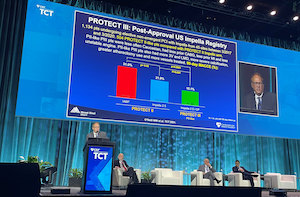
ORLANDO, Fla.–(BUSINESS WIRE)–The final results of the PROTECT III and Restore EF prospective studies demonstrate improved outcomes for high-risk PCI patients with the use of Impella heart pumps. The study results were reviewed this morning at Transcatheter Cardiovascular Therapeutics (TCT) 2021, the annual scientific symposium of the Cardiovascular Research Foundation, by Gregg […]
Final Results from the National Cardiogenic Shock Initiative (NCSI) Study Demonstrate the Benefit of Early Cardiac Unloading with Impella
DANVERS, Mass.–(BUSINESS WIRE)–The final results of the physician-led National Cardiogenic Shock Initiative (NCSI) Study demonstrate a 71% survival to discharge with greater than 90% native heart recovery when best practices are used, including placement of an Impella heart pump prior to revascularization (PCI). The study evaluated the outcomes of 406 consecutive patients who presented […]
First Patient Enrolled in PROTECT IV Randomized Controlled Trial of Impella
DANVERS, Mass.–(BUSINESS WIRE)–Abiomed (NASDAQ:ABMD) announced today that the first patient has been enrolled in PROTECT IV, a large, prospective, multi-center randomized controlled trial (RCT) that is designed to provide the level of clinical evidence needed to achieve a Class I guideline recommendation for Impella in high-risk percutaneous coronary intervention (HRPCI). The first patient […]
Data Presented at TCT Connect Finds Pre-PCI Use of Impella for AMI Cardiogenic Shock is Associated with Higher Survival, Particularly in Women
DANVERS, Mass.–(BUSINESS WIRE)–Two studies of AMI cardiogenic shock (AMICS) patients found higher survival when Impella was placed pre-PCI, compared to when Impella was placed after PCI. The findings were presented at TCT Connect, the 32nd annual scientific symposium of the Cardiovascular Research Foundation. In the first study, presented by Hemindermeet Singh, […]
Largest Study of Hemodynamically Supported High-Risk PCI Patients Finds More Complete Revascularization with Impella Leads to Improved Outcomes
DANVERS, Mass.–(BUSINESS WIRE)–Abiomed (NASDAQ: ABMD) announces new PROTECT III study data that demonstrates reduced rates of MACCE (composite of death, stroke, myocardial infarction and repeat procedures) when Impella is used to achieve a more complete revascularization in a single setting for high-risk percutaneous coronary intervention (PCI) patients. PROTECT III is an ongoing, prospective, […]
Restore EF Study Demonstrates Impella-Supported High-Risk PCI Improves Left Ventricular Ejection Fraction
DANVERS, Mass.–(BUSINESS WIRE)–The Restore EF Study demonstrates the use of contemporary best practices, including attempting a more complete revascularization with Impella-supported high-risk PCI, is associated with significant improvement of left ventricular ejection fraction (LVEF), heart failure symptoms, and anginal symptoms at follow up. The interim analysis was presented today by Mitul […]
FDA Issues Emergency Use Authorization for Impella Heart Pumps to Provide Unloading Therapy to COVID-19 Patients
DANVERS, Mass.–(BUSINESS WIRE)–The United States Food and Drug Administration (FDA) has issued an emergency use authorization (EUA) for left-sided Impella heart pumps to provide left ventricular unloading and support to COVID-19 patients who are undergoing ECMO treatment and develop pulmonary edema or myocarditis. Impella is manufactured by Abiomed (NASDAQ: ABMD). COVID-19 causes widespread inflammation which can […]
Large Multi-Center Study in Japan Finds High Survival Rates with Use of Impella Heart Pump
Study conducted at 109 hospitals with oversight by 10 Japanese professional societies, including Japanese Circulation Society TOKYO–(BUSINESS WIRE)–A three-year, investigator-led, prospective study of Japanese patients who received an Impella heart pump finds use of Impella is associated with a 77% survival rate at 30 days in AMI cardiogenic shock patients. The historic […]
Abiomed Expands Product Portfolio with Acquisition of Cardiopulmonary Support Technology (ECMO) to Improve Outcomes for Patients
DANVERS, Mass.–(BUSINESS WIRE)–Abiomed (NASDAQ: ABMD), maker of the Impella heart pump, has acquired Breethe, developer of a novel extracorporeal membrane oxygenation (ECMO) system that will complement and expand Abiomed’s product portfolio to more comprehensively serve the needs of patients whose lungs can no longer provide sufficient oxygenation, including patients suffering from cardiogenic shock […]