BOSTON–(BUSINESS WIRE)–A pivotal, multi-center clinical trial to explore a promising new therapy to reduce heart failure rates by changing the way heart attack patients are treated is now underway. The first patient has enrolled in the ST-Elevation Myocardial Infarction Door-to-Unloading (STEMI DTU) Pivotal Randomized Controlled Trial. The first enrollment took […]
Tag: Impella
First Patient Enrolls in STEMI DTU Randomized Controlled FDA Trial; Study Aims to Further Demonstrate Impella’s Safety and Effectiveness
DANVERS, Mass.–(BUSINESS WIRE)–Abiomed (NASDAQ: ABMD) announces initiation of the ST-Elevation Myocardial Infarction Door-to-Unloading (STEMI DTU) Pivotal Randomized Controlled Trial (RCT), which will explore whether unloading the heart’s left ventricle for 30 minutes with an Impella heart pump prior to opening blocked arteries will reduce infarct size after a heart attack and lead to […]
Clinical Review Demonstrates Cost-Effectiveness of Impella in High-Risk PCI and Cardiogenic Shock
DANVERS, Mass.–(BUSINESS WIRE)–To mark the five year anniversary of the study by Stretch, et al., on cost and outcomes trends for short-term mechanical circulatory support, Abiomed announces a comprehensive publication review of cost and comparative effectiveness of Impella in high-risk PCI and cardiogenic shock. The data, from a robust body of US and European […]
Abiomed Announces 1,000th Patient Treated with Impella in Japan
TOKYO–(BUSINESS WIRE)–Abiomed (NASDAQ: ABMD), a leading provider of breakthrough heart support technologies, announces the 1,000th patient has been treated with the Impella heart pump in Japan. The Impella 2.5 and Impella 5.0 heart pumps are approved for the treatment of drug-resistant acute heart failure and are the first and only percutaneous temporary […]
Abiomed Receives FDA PMA Approval for Impella 5.5 with SmartAssist, a Minimally Invasive, Forward Flow Heart Pump
DANVERS, Mass.–(BUSINESS WIRE)–Abiomed’s (NASDAQ: ABMD) newest heart pump, the Impella 5.5 with SmartAssist, has received U.S. Food and Drug Administration (FDA) pre-market approval (PMA) for safety and efficacy in the therapy of cardiogenic shock for up to 14 days. Impella 5.5 with SmartAssist is: Minimally invasive, eliminating the need for a […]
National Cardiogenic Shock Initiative (NCSI) with Impella Best Practices Demonstrates 72% Survival with 98% Native Heart Recovery
LAS VEGAS–(BUSINESS WIRE)–Abiomed (NASDAQ:ABMD) announces that new data from the National Cardiogenic Shock Initiative Study (NCSI) on 171 consecutive AMI cardiogenic shock (AMICS) patients from 35 sites demonstrates 72% survival with 98% native heart recovery at discharge. The patients were treated with the NCSI protocol, which includes placing the Impella heart pump before […]
Impella RP Post-Approval Study Data Presented at ACC 2019
March 18, 2019 07:00 AM Eastern Daylight Time NEW ORLEANS, La.–(BUSINESS WIRE)–Abiomed (NASDAQ:ABMD), a leading provider of breakthrough heart support technologies and the maker of the Impella RP heart pump, announces that survival data from the 18 month post-approval study of 42 Impella RP patients was presented at the American College of Cardiology’s […]
$100 Million Invested in Clinical Research for Impella
DANVERS, Mass.–(BUSINESS WIRE)–In a milestone for scientific research, Abiomed (NASDAQ: ABMD) has now invested more than $100 million over the past five years in clinical research on the Impella heart pump platform. Abiomed’s commitment to clinical research is detailed on a new webpage that launched today. Abiomed-sponsored research is augmented by two decades of independent physician-led […]
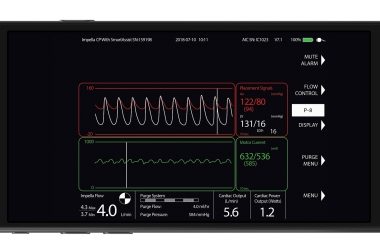
Impella Connect Achieves Regulatory Milestone
DANVERS, Mass.–(BUSINESS WIRE)–Abiomed (NASDAQ:ABMD) has achieved CE Mark for Impella Connect, the first-of-its kind cloud-based technology that enables secure, real-time, remote viewing of the Impella console for physicians and hospital staff from anywhere with Internet connectivity. European CE Mark adds to Impella Connect’s previous U.S. FDA PMA approval. Impella Connect uses […]
Data Presented at TCT 2018 Shows Use of Impella and Best Practices Increases Cardiogenic Shock Survival
SAN DIEGO, Sept. 24, 2018 (GLOBE NEWSWIRE) — A new analysis of data from Abiomed’s (NASDAQ:ABMD) Impella® Quality (IQ) Database shows a relative increase of 24% in mean survival in acute myocardial infarction (AMI) cardiogenic shock patients since Impella’s cardiogenic shock FDA post-market approval. Part of the reason for the increase was a […]