A Pivotal Step in Optimizing the ADAPT 2.0 Technique for Speed and Simplicity with a Single System Setup ADAPT 2.0 with the Zoom Stroke System CAMPBELL, Calif.–(BUSINESS WIRE)–Imperative Care, Inc. today announced the commercial launch and first patient cases of the new Zoom 4S Catheter – an advanced aspiration thrombectomy […]
Tag: Imperative Care
Imperative Care Presents Positive Real-World Data from Ischemic Stroke Patients Treated with ADAPT 2.0 Using the Zoom Stroke System
Late-Breaking Data Presented at Society of Vascular and Interventional Neurology Annual Meeting Demonstrated Excellent Reperfusion and Rapid Procedure Times with the Zoom Stroke System Using Large-Bore Intracranial Access, Asymmetric Aspiration, and Continuous Dual Aspiration CAMPBELL, Calif.–(BUSINESS WIRE)–Imperative Care, Inc. today announced the presentation of late-breaking, real-world data from a multi-center review […]
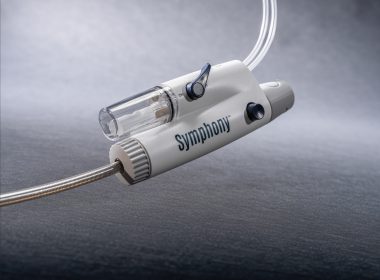
Imperative Care Announces Late-Breaking Data Presentation and Peer-Reviewed Publication from Pivotal Trial of Symphony Thrombectomy System in Patients with Acute Pulmonary Embolism
SYMPHONY-PE Trial Demonstrated Strong Efficacy, Efficiency, and Safety Results with No Mortality and No Device-Related Serious Adverse Events CAMPBELL, Calif.–(BUSINESS WIRE)–Imperative Care, Inc. today announced positive efficacy and safety results from the pivotal SYMPHONY-PE Trial (NCT06062329) evaluating the company’s Symphony® Thrombectomy System in the treatment of acute pulmonary embolism (PE). Results […]
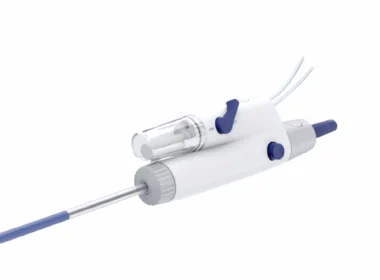
Imperative Care Announces FDA Clearance of Symphony Thrombectomy System, the First Large-Bore Continuous Vacuum System for Treatment of Pulmonary Embolism
CAMPBELL, Calif.–(BUSINESS WIRE)–Imperative Care, Inc. today announced U.S. Food and Drug Administration (FDA) 510(k) clearance of its Symphony® Thrombectomy System to treat pulmonary embolism (PE), a life-threatening condition caused by blood clots blocking an artery in the lungs. This clearance expands the use of Symphony – previously for the treatment of […]
Imperative Care Secures FDA Clearance and Initial Cases of the Zoom 7X Catheter, the Newest Innovation in Ischemic Stroke Treatment
CAMPBELL, Calif.–(BUSINESS WIRE)–Imperative Care, Inc. today announced U.S. Food and Drug Administration (FDA) 510(k) clearance and first patient cases with the latest advancement in ischemic stroke treatment – the novel Zoom 7X Catheter for aspiration thrombectomy procedures. The newest addition to the company’s comprehensive Zoom Stroke System, the Zoom 7X Catheter […]
Imperative Care Presents Positive Imperative Trial Data from Patients Treated with Aspiration Thrombectomy for M2 Occlusions Using the Zoom System
Late-Breaking Data Presented at Society of NeuroInterventional Surgery Annual Meeting Demonstrate Excellent Reperfusion Results and Unparalleled Safety with the Zoom System for Ischemic Stroke CAMPBELL, Calif.–(BUSINESS WIRE)–Imperative Care, Inc. today announced a late-breaking abstract with data from the Imperative Trial evaluating aspiration with the Zoom System in stroke patients with M2 […]
Imperative Care Announces Completion of Enrollment in the SYMPHONY-PE IDE Study for Treatment of Pulmonary Embolism
Pivotal trial evaluates safety and efficacy of the Symphony Thrombectomy System for the removal of blood clots from the lungs CAMPBELL, Calif.–(BUSINESS WIRE)–Imperative Care, Inc. today announced the completion of patient enrollment in its SYMPHONY-PE Study (NCT06062329), a pivotal Investigational Device Exemption (IDE) trial evaluating the safety and efficacy of […]
Imperative Care Launches its Next Innovation to Elevate Ischemic Stroke Treatment and Announces First Patients Treated with Continuous Dual Aspiration Technique
CAMPBELL, Calif. – Imperative Care, Inc. today announced the Zoom Stroke System has been further expanded with the launch of the Zoom DuoPort technology, which allows physicians to use the novel Continuous Dual Aspiration Technique (CDAT) on two Zoom Aspiration Catheters simultaneously from a single vacuum source. CDAT empowers physicians with enhanced […]
Imperative Care Announces Appointment of Todd Van Horn to Chief Financial Officer
CAMPBELL, Calif.–(BUSINESS WIRE)–Imperative Care, Inc. today announced the appointment of Todd Van Horn as Chief Financial Officer. “Imperative Care is deeply committed to bringing together a strong balance of leaders with complementary expertise and a shared passion for elevating patient care,” said Fred Khosravi, CEO of Imperative Care. “We are […]
Imperative Care Expands Symphony Precision Thrombectomy Portfolio with Purpose-Built Design for the Treatment of Venous Thrombosis
CAMPBELL, Calif.–(BUSINESS WIRE)–Imperative Care, Inc. today announced U.S. Food and Drug Administration (FDA) 510(k) clearance of the 82cm version of its Symphony™ 16F Catheter, the company’s latest innovation designed to elevate care for patients with venous thrombosis, a serious condition caused by a blood clot forming in the veins of […]