IRVINE, Calif., Nov. 02, 2022 (GLOBE NEWSWIRE) — Inari Medical, Inc. (NASDAQ: NARI) (“Inari”), a medical device company with a mission to treat and transform the lives of patients suffering from venous and other diseases, today reported financial results for its third quarter ended September 30, 2022. Third Quarter Revenue […]
Tag: Inari Medical
Inari Medical to Announce Third Quarter 2022 Financial Results
IRVINE, Calif., Oct. 06, 2022 (GLOBE NEWSWIRE) — Inari Medical, Inc. (NASDAQ: NARI) (“Inari”), a medical device company focused on developing products to treat and transform the lives of patients suffering from venous and other diseases, announced today that it will release its third quarter 2022 financial results on Wednesday, […]
Inari Medical Announces Results from the Fully Enrolled 800-patient US Cohort of the FlowTriever FLASH Registry
IRVINE, Calif., Sept. 19, 2022 (GLOBE NEWSWIRE) — Inari Medical, Inc. (NASDAQ: NARI) (“Inari”) a medical device company focused on developing products to treat and transform the lives of patients suffering from venous and other diseases, announced positive outcomes of the fully enrolled 800-patient FLASH registry in pulmonary embolism (“PE”). […]
Inari Medical Announces Randomized Controlled Trial Evaluating Clinical Outcomes of the ClotTriever® System in Deep Vein Thrombosis
IRVINE, Calif., Aug. 30, 2022 (GLOBE NEWSWIRE) — Inari Medical, Inc. (NASDAQ: NARI) (“Inari”) a medical device company with a mission to treat and transform the lives of patients suffering from venous and other diseases, announced planned enrollment of the DEFIANCE trial. DEFIANCE is a randomized controlled trial (“RCT”) comparing […]
Inari Medical Reports First Quarter 2022 Financial Results
IRVINE, Calif., May 04, 2022 (GLOBE NEWSWIRE) — Inari Medical, Inc. (NASDAQ: NARI) (“Inari”), a medical device company with a mission to treat and transform the lives of patients suffering from venous and other diseases, today reported financial results for its first quarter ended March 31, 2022. First Quarter Revenue […]
Inari Medical Announces First Patient Enrolled in the PEERLESS Trial, a Randomized Controlled Trial Evaluating Outcomes of the FlowTriever® System in Pulmonary Embolism Patients
IRVINE, Calif., Feb. 15, 2022 (GLOBE NEWSWIRE) — Inari Medical, Inc. (NASDAQ: NARI) (“Inari”) today announced that the first patient has been enrolled in PEERLESS, a prospective, randomized controlled trial (“RCT”) comparing the outcomes of patients with intermediate-high risk pulmonary embolism (“PE”) treated with the FlowTriever system versus catheter-directed thrombolysis […]
Inari Medical Announces Randomized Controlled Trial Evaluating Clinical Outcomes of the FlowTriever® System in Pulmonary Embolism Patients
IRVINE, Calif., Oct. 18, 2021 (GLOBE NEWSWIRE) — Inari Medical, Inc. (NASDAQ: NARI) (“Inari”) a medical device company focused on developing products to treat and transform the lives of patients suffering from venous diseases, announced planned enrollment of the PEERLESS trial. PEERLESS is a new randomized controlled trial (RCT) comparing […]
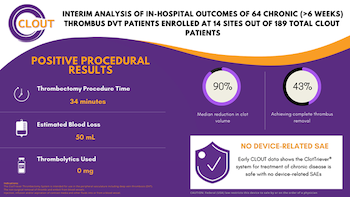
Inari Medical Announces Presentation of Positive Chronic Clot Subanalysis Results from Real World CLOUT Registry at LINC 2021
IRVINE, Calif., Jan. 25, 2021 (GLOBE NEWSWIRE) — Inari Medical, Inc. (NASDAQ: NARI) (“Inari”) a commercial-stage medical device company focused on developing products to treat and transform the lives of patients suffering from venous diseases, today announced strongly positive interim results of the first 64 chronic deep vein thrombosis (“DVT”) […]
Inari Medical Announces Presentation of Positive 30-Day Follow-Up Results from First Patients in Real World FLASH Registry
IRVINE, Calif., Nov. 13, 2020 (GLOBE NEWSWIRE) — Inari Medical, Inc. (NASDAQ: NARI) (“Inari”), a commercial-stage medical device company focused on developing products to treat and transform the lives of patients suffering from venous diseases, today announced follow-up results of the first 230 patients enrolled in its FLASH study. FLASH […]
Inari Medical Announces Completion of $27 Million Series C Financing
IRVINE, Calif., March 29, 2018 /PRNewswire/ — Inari Medical, Inc., announced today the close of a Series C financing totaling $27.0 million. The financing was led by new investor Gilde Healthcare and was joined by all of Inari’s existing investors including Versant Ventures and U.S. Venture Partners. Geoff Pardo of Gilde Healthcare will join Inari’s Board […]