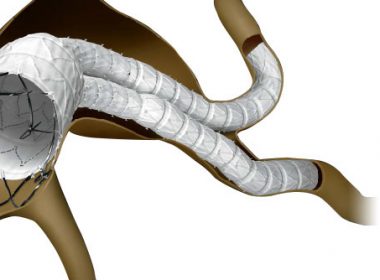
DUBLIN, Ohio, November 28, 2018 — Cordis, a Cardinal Health company (NYSE: CAH), today announced that the U.S. Food and Drug Administration (FDA) has approved its INCRAFT® AAA Stent Graft System for use in complex access anatomies. The INCRAFT system is an ultra-low profile and flexible endovascular aneurysm repair (EVAR) system designed […]
Tag: INCRAFT
FDA Advisory Committee Votes in Favor of Cardinal Health’s INCRAFT® AAA Stent Graft System for the Endovascular Treatment of Infrarenal Abdominal Aortic Aneurysms
DUBLIN, Ohio, June 12, 2018 /PRNewswire/ — Cardinal Health (NYSE: CAH) today announced that the U.S. Food and Drug Administration (FDA) Circulatory System Devices Panel of the Medical Devices Advisory Committee has provided a favorable recommendation on the premarket approval application for INCRAFT® AAA Stent Graft System (INCRAFT). The panel voted 11 to 4 in […]