Study Investigates Use of the Tack Endovascular System® for Focal Dissection Repair Following Drug-Coated Balloon Angioplasty in PAD Patients WAYNE, Pa.–(BUSINESS WIRE)–Intact Vascular, Inc., a developer of medical devices for minimally invasive peripheral vascular procedures, today announced that the one-year data from its Tack Optimized Balloon Angioplasty (TOBA) III clinical trial will be presented during […]
Tag: Intact Vascular
Journal of Endovascular Therapy Publishes Peer Reviewed Article Underscoring the Clinical Impact of Post-Angioplasty Dissections
WAYNE, Pa.–(BUSINESS WIRE)–Intact Vascular, Inc., a developer of medical devices for minimally invasive peripheral vascular procedures, today welcomed the peer-reviewed publication, “Dissections After Infrainguinal Percutaneous Transluminal Angioplasty (PTA): Systematic Review and Current State of Clinical Evidence” currently available online, with the print article scheduled to be published in the August issue […]
Intact Vascular Announces $25 Million Financing with Vensana Capital to Drive Commercialization of the Tack Endovascular System®
WAYNE, Pa.–(BUSINESS WIRE)–Intact Vascular, Inc., a developer of medical devices for minimally invasive peripheral vascular procedures, today announced a $25 million financing with Vensana Capital as the lead investor. Vensana was joined by existing investors including New Enterprise Associates (“NEA”), H.I.G. BioHealth Partners, and Quaker Partners. The Company reported closure […]
Intact Vascular to Sponsor Post-Angioplasty Dissection Symposium at 2019 New Cardiovascular Horizons Conference
WAYNE, Pa.–(BUSINESS WIRE)–Intact Vascular, Inc., a developer of medical devices for minimally invasive peripheral vascular procedures, today announced the schedule of key presentations to be featured during a lunch symposium at the 2019 New Cardiovascular Horizons (NCVH) Conference, May 29th through 31st at The Roosevelt New Orleans in New Orleans, Louisiana. Recently FDA-approved […]
Intact Vascular Announces US Launch of the Tack Endovascular System® and First Commercial Use
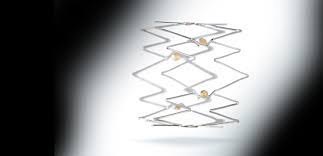
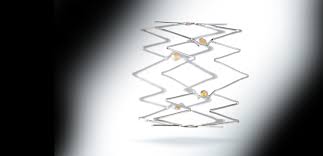
WAYNE, Pa.–(BUSINESS WIRE)–Intact Vascular, Inc., a developer of medical devices for minimally invasive peripheral vascular procedures, today announced the first commercial use of its Tack Endovascular System in multiple sites across the United States. Recently FDA-approved for above-the-knee interventions, the first-of-its-kind dissection repair device is implanted post-angioplasty to resolve dissections […]
Intact Vascular’s Tack Endovascular System® Receives FDA Approval
WAYNE, Pa.–(BUSINESS WIRE)–Intact Vascular, Inc., a private medical device company committed to developing solutions for minimally invasive peripheral vascular procedures, today announced it received U.S. Food and Drug Administration (FDA) approval for the Tack Endovascular System (6F), a purpose-built dissection repair device implanted post-angioplasty in patients with peripheral arterial disease (PAD). […]
Intact Vascular Announces Presentations at LINC 2019 Annual Meeting
WAYNE, Pa.–(BUSINESS WIRE)–Intact Vascular, Inc., a private medical device company committed to developing solutions for minimally invasive peripheral vascular procedures, today announced the schedule of key presentations to be featured at the annual Leipzig Interventional Course (LINC) in Leipzig, Germany on Tuesday, January 22nd and Wednesday, January 23rd. Intact Vascular’s Tack Endovascular […]
Intact Vascular Announces Launch of the Tack Endovascular System® in the EU and First Commercial Use in Germany
WAYNE, Pa.–(BUSINESS WIRE)–Intact Vascular, Inc., a developer of medical devices for minimally invasive peripheral vascular procedures, today announced the first commercial use of its Tack Endovascular System® in multiple hospitals within Germany. A novel therapy for dissection repair following balloon angioplasty, the Tack® implant is a first-of-its kind device for patients with peripheral […]
Intact Vascular Announces Enrollment Completion of TOBA II BTK Clinical Trial
WAYNE, Pa.–(BUSINESS WIRE)–Intact Vascular, Inc., a developer of medical devices for minimally invasive peripheral vascular procedures, today announced its Tack Optimized Balloon Angioplasty II BTK (TOBA II BTK) clinical trial successfully completed enrollment, ahead of schedule. This study further augments Intact Vascular’s robust clinical program and is notably the first pivotal trial investigating a […]
Intact Vascular to Sponsor Post-Angioplasty Dissection Symposium and Present an Update on the TOBA II Pivotal Study Results at the 2018 VEITH Conference
WAYNE, Pa.–(BUSINESS WIRE)–Intact Vascular, Inc., a private medical device company committed to developing solutions for minimally invasive peripheral vascular procedures, today announced the schedule of key presentations to be featured during a lunch symposium co-sponsored with Philips at the 45th annual VEITH conference in New York City on Thursday, November 15th. […]