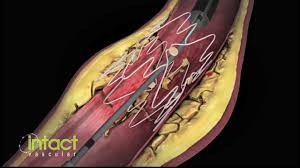
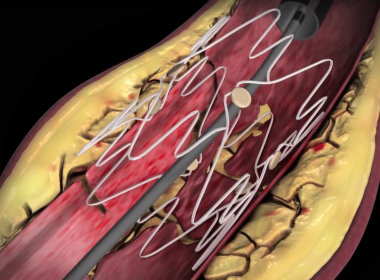
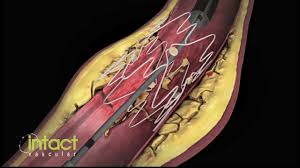
WAYNE, Pa.–(BUSINESS WIRE)–Intact Vascular, Inc., a developer of medical devices for minimally invasive peripheral vascular procedures, today announced at the 15th annual VIVA conference in Las Vegas that its Tack Optimized Balloon Angioplasty II (TOBA II) clinical trial successfully achieved both primary and secondary endpoints. One year results from the TOBA II study were presented […]
Tag: Intact Vascular
Intact Vascular Hires Industry Veteran Steve MacKinnon as Vice President of Sales
WAYNE, Pa.–(BUSINESS WIRE)–Intact Vascular, Inc., a developer of medical devices for minimally invasive peripheral vascular procedures, today announced that Steve MacKinnon has joined its senior management team as Vice President of Sales, effective immediately. Reporting to the CEO, Mr. MacKinnon will oversee the creation of a U.S. based sales team […]
Symposium to Explore the Impact of Vascular Dissections on Critical Limb Ischemia Patients at AMP 2018
WAYNE, Pa.–(BUSINESS WIRE)–Intact Vascular, Inc., a developer of medical devices for minimally invasive peripheral vascular procedures, today announced key presentations that will be featured during a CME symposium at the Amputation Prevention (AMP) Conference in Chicago on August 8. The symposium, titled “Why Dissections Matter: A case-based look at below-the-knee […]
Intact Vascular Welcomes Publication of iDissection Classification Study Results in Journal of Invasive Cardiology
WAYNE, Pa.–(BUSINESS WIRE)–Intact Vascular, Inc., a developer of medical devices for minimally invasive peripheral vascular procedures, today welcomed the publication of the iDissection Classification trial results in the current issue of Journal of Invasive Cardiology. Post-percutaneous transluminal angioplasty (“PTA”) dissections are often overlooked, underdiagnosed and left untreated. These dissections can compromise […]
Intact Vascular Announces $20 Million Series C Financing to Fund Company through PMA Approval of the Tack Endovascular System®
WAYNE, Pa.–(BUSINESS WIRE)–Intact Vascular, Inc., a developer of medical devices for minimally invasive peripheral vascular procedures, today announced that it closed a Series C financing totaling $20 million. This financing is designed to fund the Company through Pre-Market Approval (“PMA”) of the Tack Endovascular System for the treatment of post-angioplasty […]
Intact Vascular Welcomes TOBA BTK Trial One-Year Clinical Study Results Publication in Catheterization and Cardiovascular Intervention
WAYNE, Pa.–(BUSINESS WIRE)–Intact Vascular, Inc., a developer of medical devices for minimally invasive peripheral vascular procedures, today welcomed the publication of the Tack Optimized Balloon Angioplasty Below the Knee (TOBA BTK) clinical trial results in the current issue of Catheterization and Cardiovascular Intervention. This multi-center pilot study focused on collecting data supporting the safety and performance of the […]
TOBA II BTK Trial Enrollment Ahead of Schedule for the Tack Endovascular System® in Below the Knee Disease
WAYNE, Pa.–(BUSINESS WIRE)–Intact Vascular, Inc., a developer of medical devices for minimally invasive peripheral vascular procedures, today announced the Tack Optimized Balloon Angioplasty II Below the Knee (TOBA II BTK) clinical trial surpassed the 100 patient milestone, advancing enrollment well ahead of schedule. The TOBA II BTK is a prospective, […]
Positive Twenty-Four Month Tack Optimized Balloon Angioplasty (“TOBA”) Single Center Results Presented at VEITHsymposium™ Conference
WAYNE, Pa.–(BUSINESS WIRE)–Intact Vascular, Inc., a developer of medical devices for minimally-invasive peripheral vascular procedures, today announced that positive single center twenty-four month results from its Tack Optimized Balloon Angioplasty (TOBA) clinical study were presented at the VEITHsymposium™ 2017 conference by Dr. Christian Wissgott, Assistant Director, at Westküstenklinikum Heide in Heide, Germany. The TOBA study enrolled […]
Intact Vascular Announces Enrollment Of First European Patient In Tack Optimized Balloon Angioplasty II Below The Knee (TOBA II BTK) Clinical Trial With The Tack Endovascular System
WAYNE, Pa.–(BUSINESS WIRE)–Intact Vascular, Inc., a developer of medical devices for minimally invasive peripheral vascular procedures, today announced that its Tack Optimized Balloon Angioplasty II Below the Knee (TOBA II BTK) clinical trial has commenced enrollment in Europe, with the first patient treated by Professor Dr. Marianne Brodmann and Dr. Peter Reif at Medical University Graz, Austria. […]
Intact Vascular Release: FDA Approves 6-Month Primary Endpoint For The Tack Endovascular System In Below The Knee Disease
WAYNE, Pa.–(BUSINESS WIRE)–Intact Vascular, Inc., a developer of medical devices for minimally invasive peripheral vascular procedures, today announced the U.S. Food and Drug Administration (FDA) approved an Investigational Device Exemption (IDE) supplemental application to modify the primary endpoint in the Tack Optimized Balloon Angioplasty II Below the Knee (TOBA II BTK) clinical trial from 12 months to […]