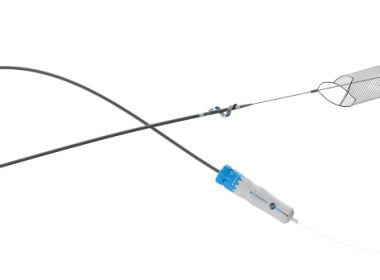
REDWOOD CITY, Calif.–(BUSINESS WIRE)–InterVene, Inc., a privately held medical device company pioneering interventional devices for venous occlusions, today announced it received 510(k) clearance from the U.S. Food and Drug Administration (FDA) for the Recana® Thrombectomy Catheter System for treating venous in-stent restenosis and native vessel obstructions. Recana is a fully […]
Tag: InterVene
InterVene, Inc. Announces $13 Million Series A Financing to Advance Recana™ System Through US Market Clearance
October 07, 2024 08:00 AM Eastern Daylight Time REDWOOD CITY, Calif.–(BUSINESS WIRE)–InterVene, Inc., a clinical-stage medical device company dedicated to developing interventional solutions for chronic venous insufficiency (CVI), announced today the closing of its $13M Series A financing round. The financing was co-led by new investor Treo Ventures and existing […]
InterVene’s BlueLeaf Endovenous Valve Formation (EVF) System Granted Breakthrough Device Designation by the FDA
SOUTH SAN FRANCISCO, Calif.–(BUSINESS WIRE)–InterVene Inc. announced today that it has received Breakthrough Device Designation by the FDA for the company’s BlueLeaf® Endovenous Valve Formation System. BlueLeaf is the first catheter-based solution developed for deep vein reflux — the failure of venous valves in the legs — which does not require […]
InterVene Raises $15 Million in Series B Funding for First Catheter-Based System to Correct Deep Vein Valve Failure
SOUTH SAN FRANCISCO–(BUSINESS WIRE)–InterVene Inc. today announced it has raised $15 million in a Series B financing round. The company’s BlueLeaf® Endovenous Valve Formation System is the first catheter-based solution for deep vein reflux (DVR) — the failure of venous valves in the legs — that does not require an implant. […]