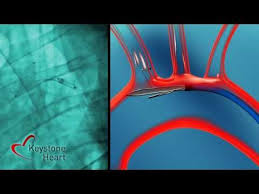
TAMPA, Fla., Oct. 27, 2021 /PRNewswire/ — Keystone Heart Ltd., a Venus Medtech Company, today announced a key milestone in Europe with the TriGUARD 3 Cerebral Embolic Protection (CEP) Device. The device was granted CE mark in March of 2020 and, since launch, utilized in fourteen countries with zero physician-reported strokes. The TriGUARD 3 CEP device is the only […]
Tag: Keystone Heart
Keystone Heart Ltd. and InterValve Medical Inc. enter distribution agreement for balloon aortic valvuloplasty portfolio
TAMPA, Fla., March 25, 2021 /PRNewswire/ — Keystone Heart, Ltd., a medical device innovator driven to redefine the standard of structural heart care, today announced an exclusive distribution agreement with InterValve Medical Inc. to sell and market their portfolio of balloon aortic valvuloplasty products, in the United States. InterValve Medical, a Venus Medtech […]
TriGUARD 3™ Cerebral Embolic Protection Device Receives CE Mark for Use in Transcatheter Heart Procedures
TAMPA, FL March 10, 2020- Keystone Heart Ltd., a Venus Medtech Company, today announced European CE Mark for the TriGUARD 3™ Cerebral Embolic Protection (CEP) Device. The device is designed to minimize the risk of cerebral damage by deflecting embolic debris away from cerebral circulation during Transcatheter Aortic Valve Implantation […]
Venus Medtech Completes Merger with Keystone Heart, LTD
HANGZHOU, China and CAESAREA, Israel and TAMPA, Fla., Dec. 26, 2018 /PRNewswire/ — Venus Medtech (Hangzhou) Inc., the preeminent Chinese transcatheter heart valve company, today announced it has closed its Merger with Keystone Heart Ltd., a privately-held medical device company and makers of TriGUARD 3™. The Merger provides Venus Medtech with TriGUARD 3, the first Cerebral Embolic Protection Device designed […]
Venus Medtech Announces Agreement to Acquire Keystone Heart, LTD
HANGZHOU, China, CAESAREA, Israel, TAMPA, Florida — September 25, 2018— Venus Medtech (Hangzhou) Inc., the preeminent Chinese transcatheter heart valve company, today announced it has signed an agreement to acquire Keystone Heart Ltd., a privately-held medical device company and makers of TriGUARD 3™. The acquisition gives Venus Medtech international rights to […]
Keystone Heart Ltd Announces First Enrollment in REFLECT Phase II incorporating TriGUARD 3™ Cerebral Embolic Protection device
CAESAREA, Israel and TAMPA, Fla., May 31, 2018 /PRNewswire/ — Keystone Heart Ltd., the leading medical device company focused on providing complete cerebral protection for patients undergoing cardiac procedures, today announced the launch of Phase II of the REFLECT trial to evaluate the safety and efficacy of the next generation of Keystone Heart […]
Venus Medtech’s strategic investment in Keystone Heart deepens the technical layout of cerebral embolic protection market
HANGZHOU, China, May 23, 2018 /PRNewswire/ — China Venus Medtech (Hangzhou) Inc. announced a partnership agreement for strategic investment and authorization with Keystone Heart. This agreement will grant Venus Medtech the right to exclusively develop, manufacture and sell the 3rd generation TriGUARD 3™ device and the next generation of cerebral embolic protection device in China and […]
Keystone Heart Ltd to Accelerate Trials for Advanced Device to Protect Brain During Heart Procedures
NEWS PROVIDED BY Keystone Heart Ltd. Aug 01, 2017, 13:06 ET CAESAREA, Israel and TAMPA, Fla., Aug. 1, 2017 /PRNewswire/ — Keystone Heart Ltd., an emerging medical device company focused on developing cerebral protection devices for patients undergoing cardiac procedures, today announced plans to initiate clinical trials for a new, advanced version of Keystone Heart TriGuard™ cerebral […]