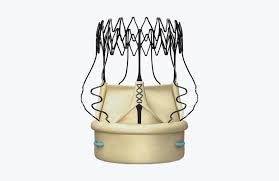
LONDON–(BUSINESS WIRE)–LivaNova PLC (NASDAQ:LIVN), a market-leading medical technology company, announced today that given the increasing viability of Valve-in-Valve (ViV) procedures, it has added new safety and technical information to the Perceval U.S. Instructions For Use (IFUs). This addition was made following completion of the Company’s application to the U.S. Food […]
Tag: LivaNova
LivaNova’s Perceval Sutureless Aortic Heart Valve Receives National Reimbursement in Japan to Treat Patients with Aortic Valve Disease
LONDON–(BUSINESS WIRE)–LivaNova PLC (NASDAQ:LIVN), a market-leading medical technology company, today announced that Japan’s Ministry of Health, Labour and Welfare granted national reimbursement for the Company’s Perceval® sutureless aortic heart valve to treat aortic valve disease. By adding Perceval into Japan’s health insurance system, physicians and patients have greater access to this […]
New Studies in the U.S. and Europe Demonstrate LivaNova Perceval Valve’s Strong Performance and Economic Advantage in Aortic Disease Treatment
LONDON–(BUSINESS WIRE)–LivaNova PLC (NASDAQ:LIVN), a market-leading medical technology company, today announced the publication of three separate studies highlighting the unique performance of its sutureless aortic valve, Perceval®. The results from the Perceval Aortic Heart Valve Study in North America, performed under a U.S. Food and Drug Administration (FDA) Investigational Device […]
LivaNova Reports Third Quarter 2018 Results
LONDON–(BUSINESS WIRE)–LivaNova PLC (NASDAQ:LIVN), a market-leading medical technology and innovation company, today reported results for the quarter ended September 30, 2018. For the third quarter of 2018, worldwide sales from continuing operations were $272.1 million, an increase of 8.3 percent on a reported basis and an increase of 10.3 percent on […]
LivaNova to Present at the Stifel 2018 Healthcare Conference
LONDON–(BUSINESS WIRE)–LivaNova PLC (NASDAQ:LIVN), a market-leading medical technology company, today announced that Damien McDonald, Chief Executive Officer, will present at the Stifel 2018 Healthcare Conference on Tuesday, Nov. 13 in New York at 9:30 a.m. Eastern Standard Time. The discussion will be available to all interested parties through a live […]
LivaNova to Host Conference Call for Third Quarter 2018 Results
LONDON–(BUSINESS WIRE)–LivaNova PLC (NASDAQ:LIVN), a market-leading medical technology company, will host a conference call to discuss its third quarter 2018 results on Wednesday, Oct. 31, 2018 at 12 p.m. London time (8 a.m. Eastern Daylight Time). The Company will release its third quarter 2018 results prior to the call. A […]
LivaNova Launches International Pivotal Study Evaluating Use of Autonomic Regulation Therapy for Heart Failure
LONDON–(BUSINESS WIRE)–LivaNova PLC (NASDAQ:LIVN), a market-leading medical technology company, today announced the first successful implantation of the VITARIA® System in a patient enrolled in the Autonomic Regulation Therapy to Enhance Myocardial Function and Reduce Progression of Heart Failure With Reduced Ejection Fraction (ANTHEM-HFrEF) Pivotal Study. The study is an international, multi-center, randomized trial to evaluate the VITARIA System for the […]
LivaNova Welcomes NICE Interventional Procedures Guidance on Sutureless Aortic Valve Replacement
LONDON–(BUSINESS WIRE)–LivaNova PLC (NASDAQ:LIVN), a market-leading medical technology company, welcomes the recently updated interventional procedures guidance for sutureless aortic valve replacement issued by the U.K. National Institute for Health and Care Excellence (NICE), which states that sutureless aortic valve replacement is an alternative to conventional surgical aortic valve replacement. The […]
LivaNova Concludes PRELUDE Study for Transcatheter Mitral Valve Replacement System
LONDON–(BUSINESS WIRE)–LivaNova PLC (NASDAQ: LIVN), a market-leading medical technology company, today announced the conclusion of the PRELUDE feasibility study for its Caisson Transcatheter Mitral Valve Replacement (TMVR) system. The PRELUDE first-in-human study evaluated the Company’s TMVR system to treat moderate to severe mitral regurgitation (MR) using a transseptal approach. This […]
LivaNova Reports Second Quarter 2018 Results
LONDON–(BUSINESS WIRE)–LivaNova PLC (NASDAQ:LIVN), a market-leading medical technology and innovation company, today reported results for the quarter ended June 30, 2018. For the second quarter of 2018, worldwide sales from continuing operations were $287.5 million, an increase of 12.4 percent on a reported basis and an increase of 10.2 percent on […]