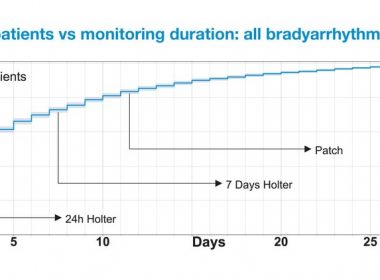
NEW ORLEANS, March 14, 2019 /PRNewswire/ — Medi-Lynx Cardiac Monitoring, LLC, a leading provider of cardiac monitoring solutions, today announced results from a large retrospective analysis which demonstrated that cardiac monitoring with full-disclosure mobile cardiac telemetry (PocketECG) for up to 30 days significantly increased diagnostic yield for patients with bradyarrhythmia over Patch and […]
Tag: Medi-Lynx
MEDICALgorithmics, Medi-Lynx Receive FDA 510(K) Clearance for PocketECG CRS Mobile Cardiac Rehabilitation System
PLANO, Texas, July 23, 2018 /PRNewswire/ — MEDICALgorithmics, S.A. (WSE: MDG) and U.S. subsidiary Medi-Lynx Cardiac Monitoring L.L.C., today announced that the U.S. Food and Drug Administration (FDA) has granted 510(k) clearance for the PocketECG Cardiac Rehabilitation System (CRS), a new mobile cardiac rehabilitation system designed to provide high-quality ECG monitoring and […]