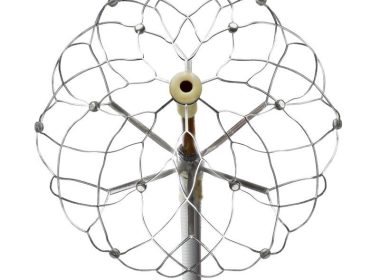
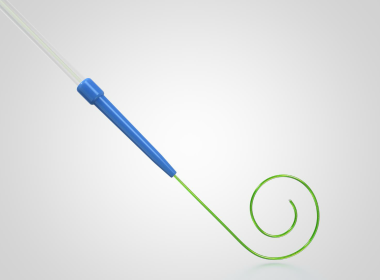
First-of-its-kind, rotation-free single-shot PFA catheter supported by strong safety and efficacy data adds to the groundbreaking Affera family of technologies in Europe Successful completion of first cases at multiple sites kicks off Horizon 360 IDE pivotal trial in the United States…
Tag: Medtronic
Medtronic reports strong second quarter fiscal 2026 financial results, enterprise growth drivers accelerate momentum
Cardiac Ablation Solutions growth of 71% on strength of pulsed field ablation (PFA) portfolio; Raising FY26 revenue and EPS guidance GALWAY, Ireland, Nov. 18, 2025 /PRNewswire/ — Medtronic plc (NYSE: MDT), a global leader in healthcare technology, today announced financial results for…
Medtronic Aurora EV-ICD™ system demonstrates exceptional results at six months in the Enlighten real-world post-market study while the OmniaSecure™ defibrillation lead sustains performance at three years in the LEADR Pivotal Trial
APHRS.25: Medtronic showcases breadth of defibrillation portfolio with outcomes from extravascular and transvenous defibrillation studies for the treatment of dangerous heart rhythm episodes Cardiac Rhythm Management Researchers presented results from two late-breaking presentations at the Asia Pacific Heart Rhythm Society (APHRS) Scientific Session 2025 held in Yokohama, Japan. The six-month […]
Medtronic announces launch of Stedi™ Extra Support Guidewire to enhance Medtronic TAVR system performance
Stedi Extra Support Guidewire Delivers Enhanced Support for Stability, Safety, and Predictability During Valve Deployment for Aortic Stenosis Medtronic plc, a global leader in healthcare technology, today announced the launch of the Stedi™ Extra Support guidewire, designed to enhance performance of the Evolut™ transcatheter aortic valve replacement (TAVR) platform […]
Six-month SPYRAL AFFIRM results from Medtronic demonstrate blood pressure reductions in high-risk cardiovascular patients
Medtronic also announced completion of enrollment in SPYRAL AFFIRM, which aims to build robust real-world evidence across traditionally underserved patient populations Medtronic plc, a global leader in healthcare technology, today announced the completion of enrollment and the first results from the High Cardiovascular Risk Patient Cohort of the SPYRAL AFFIRM […]
Medtronic receives CE Mark for Penditure™ Left Atrial Appendage (LAA) Exclusion System, ushering in a new era of left atrial appendage management in Europe as it surpasses 10,000 Penditure Devices Sold Globally
Medtronic plc, a global leader in healthcare technology, today announced its Penditure™ Left Atrial Appendage (LAA) Exclusion System has officially received CE Mark approval and will be launched in Europe at the 39th European Association for Cardio-Thoracic Surgery (EACTS) Annual Meeting from October 8-11 in Copenhagen, Denmark. The Penditure device recently […]
Medtronic initiates global pivotal study of cardiac pacing in a new patient population
Study to evaluate whether a new approach to pacing the heart can improve the lives of patients with heart failure with preserved ejection fraction (HFpEF) who have limited treatment options today GALWAY, Ireland, Sept. 15, 2025 /PRNewswire/ — Medtronic plc (NYSE: MDT), a global leader in…
Compelling data at ESC Congress 2025 reinforce safety and performance of the Medtronic OmniaSecure™ defibrillation lead, including in the left bundle branch area
The upcoming U.S. launch of the small-diameter OmniaSecure defibrillation lead will introduce the first FDA-approved lead for both adults and adolescent pediatric patients Cardiac Rhythm Management At the 2025 European Society of Cardiology (ESC) Congress in Madrid, researchers presented three new datasets reinforcing the safety and performance of the OmniaSecure™ […]
Medtronic receives approval for Symplicity Spyral™ Renal Denervation System in Japan
Japan is now the 77th country to receive regulatory approval for the Symplicity Spyral Renal Denervation System Medtronic, a global leader in healthcare technology, today announced it received approval in Japan from the Pharmaceuticals and Medical Devices Agency (PMDA) for its Symplicity Spyral™ renal denervation (RDN) system, also known as […]
Medtronic Evolut TAVR systems receive FDA approval for expanded Redo TAVR indication
Medtronic plc, a global leader in healthcare technology, today announced it received U.S. Food and Drug Administration (FDA) approval for the expanded Redo-TAVR indication of the Evolut™ transcatheter aortic valve replacement (TAVR) system. This approval allows for the implantation of a new Evolut transcatheter aortic valve (TAV) inside any failed […]