Revenue of $7.7 Billion Increased 3.0% Reported and 4.1% Organic GAAP Diluted EPS of $1.01; Non-GAAP Diluted EPS of $1.31 Cash Flow from Operations of $1.9 Billion Grew 61%; Free Cash Flow of $1.6 Billion Grew 66% Company Raises FY20 EPS Guidance DUBLIN, Nov. 19, 2019 (GLOBE NEWSWIRE) — Medtronic plc […]
Tag: Medtronic
New Study Shows Promise in Treating More Patients with World’s Smallest Pacemaker
Data Published in JACC: Clinical Electrophysiology and to be Presented at American Heart Association Scientific Sessions Demonstrates the Potential of Investigational Algorithms in Medtronic Micra Pacemaker to Improve Synchrony and Cardiac Function in AV Block Patients DUBLIN, Nov. 11, 2019 (GLOBE NEWSWIRE) — Medtronic plc (NYSE:MDT) today announced results from the MARVEL […]
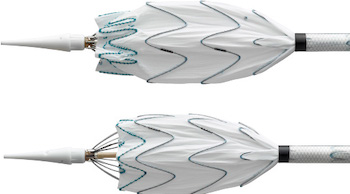
Medtronic Announces Shonin Approval and Launch of the Valiant Navion™ Thoracic Stent Graft System in Japan
Lower-Profile Thoracic Endovascular Aortic Repair (TEVAR) Device Continues to Broaden Global Treatable Patient Population with Thoracic Aortic Disease DUBLIN, Oct. 31, 2019 (GLOBE NEWSWIRE) — Medtronic plc (NYSE:MDT) today announced Shonin approval from the Ministry of Health, Labour and Welfare (MHLW) and the launch of the Valiant Navion™ thoracic stent graft system […]
Medtronic Receives FDA “Breakthrough Device Designation” for Developing Fully Implantable Heart Pump
DUBLIN, Oct. 29, 2019 (GLOBE NEWSWIRE) — Medtronic plc (NYSE:MDT), the global leader in medical technology, today announced it has received Breakthrough Device designation from the U.S. Food and Drug Administration (FDA) for the Medtronic Fully Implantable Left Ventricular Assist Device (LVAD) for patients with advanced heart failure, currently in development. […]
FDA Classifies Medtronic Sherpa Delivery Catheter Field Action Initiated in March 2019 as Class I Recall
DUBLIN, Oct. 09, 2019 (GLOBE NEWSWIRE) — Medtronic plc (NYSE:MDT) today announced the U.S. Food and Drug Administration (FDA) has classified the company’s voluntary field action initiated in March 2019 related to the 6F Sherpa™ NX Active Coronary Guide Catheter as a Class I recall. The catheter is used during coronary […]
Medtronic Receives FDA Breakthrough Device Designation for Developing Stent Graft System to Treat Thoracoabdominal Aortic Aneurysm
News Follows Medtronic Receiving FDA Breakthrough Device Designation for its Valiant Navion™ LSA Branch Thoracic Stent Graft System DUBLIN, Oct. 08, 2019 (GLOBE NEWSWIRE) — Medtronic plc (NYSE:MDT) today announced it has received Breakthrough Device designation from the U.S. Food and Drug Administration (FDA) for its Valiant® TAAA Stent Graft System […]
Medtronic Initiates Worldwide Pivotal Study of a New Approach to Treating Dangerously Fast Heart Rhythms
Study to Evaluate Novel Implantable Defibrillator System with a Lead Placed Outside the Heart and Veins DUBLIN, Oct. 07, 2019 (GLOBE NEWSWIRE) — Medtronic plc (NYSE:MDT) today announced the start of a worldwide pivotal study evaluating its investigational Extravascular Implantable Cardioverter Defibrillator (EV ICD) system to treat dangerously fast heart rhythms. The […]
Medtronic Announces Early Feasibility Trial for Intrepid™ Transcatheter Mitral Valve Replacement System with Transfemoral Transseptal Approach
New Study Shows Momentum in Establishing a Less Invasive Approach to Treat Patients with Severe Mitral Valve Disease DUBLIN, Sept. 27, 2019 (GLOBE NEWSWIRE) — Medtronic plc (NYSE:MDT) today announced it has received U.S. Food and Drug Administration (FDA) approval to begin an early feasibility study (EFS) for its Intrepid™ transcatheter mitral […]
Resolute Onyx™ DES Meets Primary Endpoint in First-Ever Clinical Study Comparing Drug-Eluting Stents in High-Bleeding Risk (HBR) Patients with One-Month DAPT
DUBLIN and SAN FRANCISCO, Sept. 26, 2019 (GLOBE NEWSWIRE) — Medtronic plc (NYSE:MDT) announced today late-breaking clinical data from the Onyx ONE Global Study, representing the first prospective, multi-center, randomized study evaluating clinical outcomes between two drug-eluting stents (DES) in nearly 2,000 high-bleeding risk (HBR) patients with one month of dual antiplatelet therapy […]
Medtronic Announces FDA Approval and U.S. Launch of Next-Generation Evolut™ PRO+ TAVR System for Treatment of Symptomatic Severe Aortic Stenosis Patients
Improved Functionality Across Four Valve Sizes and Lowest Delivery Profile on the Market, the Evolut PRO+ TAVR System Launches in U.S. as TAVR Patient Population Grows DUBLIN, Sept. 23, 2019 (GLOBE NEWSWIRE) — Medtronic plc (NYSE:MDT), a global leader in heart valve therapies, today announced U.S. Food and Drug Administration […]