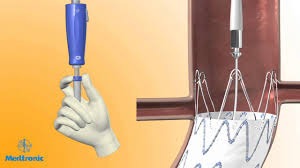
DUBLIN – September 21, 2017 – Medtronic plc (NYSE: MDT) today announced a new post-market clinical study to evaluate its CoreValve(TM) Evolut(TM) PRO valve in everyday clinical practice. Studying patients with severe symptomatic aortic stenosis at an intermediate, high or extreme risk for open heart surgery, the FORWARD PRO Clinical Study […]
Tag: Medtronic
Medtronic (MDT) Shows Off New 5-Year Subset Data From Endurant AAA Stent Graft Trial
DUBLIN and LAS VEGAS – September 13, 2017 – Medtronic plc (NYSE: MDT) today reported its Endurant® II abdominal aortic aneurysm (AAA) stent graft system continues to demonstrate long-term durability and consistent outcomes in a real-world setting among both male and female patients. The five-year ENGAGE global registry data were presented […]
Medtronic (MDT) IN.PACT Admiral DCB Global Two Year Data And IN.PACT SFA Four Year Data Presented In VIVA Late Breaking Clinical Trials
DUBLIN and LAS VEGAS – September 12, 2017 – Medtronic plc (NYSE: MDT) data announced today reinforce the durability and safety of the IN.PACT(TM) Admiral(TM) drug-coated balloon (DCB) in patients with peripheral arterial disease (PAD). The two-year, real-world results from the full clinical cohort of the IN.PACT Global Study and four-year […]
Medtronic (MDT) Announces Japanese Regulatory Approval For The IN.PACT Admiral Drug-Coated Balloon
DUBLIN – September 8, 2017 – Medtronic plc (NYSE: MDT) today announced that the IN.PACT(TM)Admiral(TM) Drug-Coated Balloon (DCB) received approval from the Japanese Ministry of Health, Labour and Welfare (MHLW) for the treatment of peripheral artery disease (PAD) in the upper leg – specifically, in the thigh (superficial femoral arteries (SFA)) and […]
Medtronic Plans for Renal Denervation Pivotal Trial After New Study Shows Significant Blood Pressure Reductions
Published Simultaneously in The Lancet, the Late-Breaking SPYRAL HTN-OFF MED Study at ESC Successfully Isolates RDN Treatment Effect to Show Compelling Efficacy and Safety of Hypertension Procedure DUBLIN and BARCELONA – August 28, 2017 – Medtronic plc (NYSE:MDT) today announced its intent to move forward with a new renal denervation pivotal trial […]
SetPoint Secures $30 Million Series D Equity Financing
Valencia, CA – August 28, 2017 – SetPoint Medical, a clinical-stage biomedical technology company developing a bioelectronic therapy for chronic inflammatory diseases, announced today that it has secured $30 million in Series D equity financing. The Series D financing will provide SetPoint with funding to further advance the clinical development of […]
Medtronic Receives CE Mark for Attain Stability(TM) Quad MRI SureScan(TM) Active-Fixation Heart Lead
DUBLIN – August 21, 2017 – Medtronic plc (NYSE: MDT) announced it has received CE (Conformité Européene) Mark for the Attain Stability(TM) Quad MRI SureScan(TM) left heart lead. Paired with Medtronic quadripolar cardiac resynchronization therapy-defibrillators (CRT-D) and -pacemakers (CRT-P), the Attain Stability Quad lead features novel, active-fixation technology that is designed […]
Minnesota Supreme Court allows shareholder suit over Medtronic-Covidien merger
Updated to clarify that MDT officers and directors were compensated for excise-tax based liabilities related to stock options, and not for capital gains. Medtronic (NYSE:MDT) shareholders seeking to sue the company claiming they were harmed by its $50 billion corporate inversion merger with Covidien will get their chance in court, the […]
Medtronic Announces Randomized Global Resolute Onyx(TM) DES One-Month Dual Antiplatelet Therapy Study to Address Critical Unanswered Question in Interventional Cardiology
DUBLIN- August 14, 2017 – Medtronic plc (NYSE: MDT) today announced a global randomized clinical trial that will evaluate one-month dual antiplatelet therapy (DAPT) – the combination of aspirin and an anti-clotting medication – in patients implanted with the Resolute Onyx(TM) Drug-Eluting Stent (DES) during percutaneous coronary intervention (PCI). Designed to […]
Medtronic (MDT) Bags Approval for Avalus Valve in U.S. and Europe
DUBLIN – August 2, 2017 – Medtronic plc (NYSE: MDT) today announced CE (Conformité Européenne) mark and U.S. Food and Drug Administration (FDA) approval of its new Avalus(TM) pericardial aortic surgical valve for the treatment of aortic valve disease. The Avalus valve leverages proven surgical bioprosthetic valve concepts with added features designed to […]