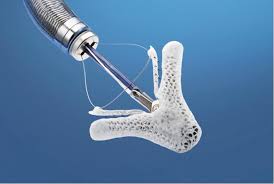
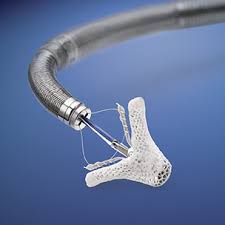
– New clinical trial will assess first-of-its-kind MitraClip™ transcatheter mitral valve repair against current standard of care surgery in a new, expanded patient population – If successful, the REPAIR MR study has potential to expand MitraClip’s current indication (patients at prohibitive risk for surgery) to also include patients at moderate […]
Tag: Mitraclip
New Data Demonstrate Strong Outcomes for Abbott Device to Repair Leaky Tricuspid Heart Valves
– New data presented at TCT 2019 on Abbott’s TriClip device from the TRILUMINATE Feasibility Study met both the primary safety and performance endpoints – Abbott’s TriClip tricuspid valve repair system design is based on the company’s market-leading MitraClip™ technology SAN FRANCISCO, Sept. 28, 2019 /PRNewswire/ — Abbott (NYSE: ABT) today announced new […]
First MitraClip™ Procedure Performed Using the All-in-One VersaCross™ Transseptal Solution
TORONTO, Sept. 23, 2019 /PRNewswire/ – Baylis Medical announced today the successful first MitraClip™ procedure performed using the VersaCross™ Transseptal Solution. The VersaCross solution is the world’s first all-in-one left-heart access device featuring an atraumatic RF-tipped pigtail wire. The device is designed to achieve left-heart access with controlled precision, while reducing the […]
Abbott Receives U.S. Approval of Next-Generation MitraClip®, Bringing New Enhancements to Abbott’s Leading MitraClip Platform
ABBOTT PARK, Ill., July 15, 2019 /PRNewswire/ — Abbott (NYSE: ABT) today announced the company has received U.S. Food and Drug Administration (FDA) approval for the most advanced MitraClip™ heart valve repair device to treat mitral regurgitation. The latest approval for the fourth-generation MitraClip device, MitraClip G4, puts new enhancements into the hands of […]
TRILUMINATE Study Shows Treatment with Abbott’s First-of-its-Kind Minimally Invasive Device Reduces Tricuspid Heart Valve Leaks
PARIS, May 21, 2019 /PRNewswire/ — Abbott (NYSE: ABT) today announced positive late-breaking data from its TRILUMINATE study of the company’s minimally invasive tricuspid valve repair system. Results at 30 days demonstrated that the investigational device is associated with a reduction of tricuspid regurgitation (TR) symptoms – caused by a leaky tricuspid heart valve […]
ABBOTT RECEIVES FDA APPROVAL FOR EXPANDED INDICATION FOR MITRACLIP™ DEVICE
ABBOTT PARK, Ill., March 14, 2019 /PRNewswire/ — Abbott (NYSE: ABT) today announced it received approval from the U.S. Food and Drug Administration (FDA) for a new, expanded indication to its leading MitraClip™ device used to repair a leaky mitral valve without open-heart surgery. Supported by the results of the landmark COAPT™ Trial, […]
Landmark Study Shows Treatment with Abbott’s MitraClip® is Superior to Medical Therapy for Advanced Heart Failure Patients with Significant Secondary Mitral Regurgitation
SAN DIEGO, Sept. 23, 2018 /PRNewswire/ — Abbott (NYSE :ABT ) today announced positive clinical study results from a randomized controlled trial comparing treatment with the MitraClip® device to guideline-directed medical therapy in select patients with secondary (or functional) mitral regurgitation, or a leaky heart valve, as a result of advanced heart failure. The landmark […]
Abbott Receives FDA Approval for Next-Generation MitraClip® Device to Treat People with Leaky Heart Valves
ABBOTT PARK, Ill., July 12, 2018 /PRNewswire/ — Abbott (NYSE: ABT) today announced it received approval from the U.S. Food and Drug Administration (FDA) for a next-generation version of its leading MitraClip® heart valve repair device used to repair a leaky mitral valve without open-heart surgery. The transcatheter clip-based therapy, now on a third generation […]
Abbott’s MitraClip Therapy Receives National Reimbursement in Japan to Treat Patients with Mitral Regurgitation
ABBOTT PARK, Ill., March 19, 2018 /PRNewswire/ — Abbott (NYSE: ABT) today announced that the Ministry of Health Labour and Welfare (MHLW) in Japan granted national reimbursement for the company’s MitraClip therapy to treat people with mitral regurgitation, a serious, progressive heart disease in which the mitral valve does not close properly, […]
Late-Breaking Data on Abbott’s MitraClip® System Show Continued Benefit for People with Mitral Regurgitation, Most Common Heart Valve Disease
WASHINGTON, March 18, 2017 /PRNewswire/ — Abbott (ABT) today announced favorable one-year outcomes from the largest study of real-world experience for the MitraClip system in transcatheter mitral valve repair (TMVR) procedures in the United States. MitraClip treats people with degenerative mitral regurgitation (DMR, also known as leaky heart valve), a […]