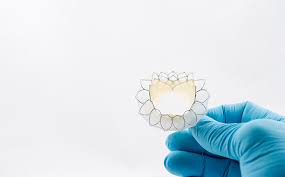
VANCOUVER, Nov. 14, 2018 /PRNewswire/ – Neovasc, Inc. (“Neovasc” or the “Company”) (NASDAQ: NVCNTSX: NVCN), a leader in the development of minimally invasive transcatheter mitral valve replacement technologies and in the development of minimally invasive devices for the treatment of refractory angina, today reported financial results for the third quarter ended September 30, 2018. “In […]
Tag: Neovasc
Neovasc Announces Third Quarter 2018 Financial Results Conference Call and Webcast
VANCOUVER, Nov. 7, 2018 /PRNewswire/ – Neovasc, Inc. (“Neovasc” or the “Company”) (NASDAQ,TSX: NVCN), a leader in the development of minimally invasive transcatheter mitral valve replacement technologies and in the development of minimally invasive devices for the treatment of refractory angina, today announced that it will report financial results for the quarter and […]
Neovasc Announces Successful Tiara™ “Live Case” at the 32nd Annual EACTS 2018 Meeting
VANCOUVER, Oct. 22, 2018 /PRNewswire/ – Neovasc Inc. (“Neovasc” or the “Company”) (NASDAQ: NVCN) (TSX: NVCN), a leader in the development of minimally invasive transcatheter mitral valve replacement technologies and in the development of minimally invasive devices for the treatment of refractory angina, today announced that its Tiara™ (“Tiara”) transcatheter mitral […]
Neovasc Announces Publication of a Peer-Reviewed Article on Tiara™ Cases in Circulation: Cardiovascular Interventions
VANCOUVER, Oct. 16, 2018 /PRNewswire/ – Neovasc Inc. (“Neovasc” or the “Company”) (NASDAQ: NVCN) (TSX: NVCN), a leader in the development of minimally invasive transcatheter mitral valve replacement technologies and in the development of minimally invasive devices for the treatment of refractory angina, today announced that an original article was published in Circulation: Cardiovascular Interventions titled […]
Neovasc Reducer™ Granted Breakthrough Device Designation from FDA
VANCOUVER, Oct. 10, 2018 /PRNewswire/ – Neovasc Inc. (“Neovasc” or the “Company”) (NASDAQ: NVCN) (TSX: NVCN), a leader in the development of minimally invasive transcatheter mitral valve replacement technologies and in the development of minimally invasive devices for the treatment of refractory angina, today announced that the U.S. Food and Drug Administration (the “FDA”) […]
Neovasc Announces Effective Date of Share Consolidation
VANCOUVER, Sept. 18, 2018 /PRNewswire/ – Neovasc Inc. (“Neovasc” or the “Company“) (NASDAQ :NVCN )(TSX :NVCN ), a leader in the development of minimally invasive transcatheter mitral valve replacement technologies and in the development of minimally invasive devices for the treatment of refractory angina, announced today that the Company has filed articles of amendment, effective […]
Neovasc Announces Extension to Regain Compliance with Nasdaq Minimum Bid Price Rule
VANCOUVER, Sept. 11, 2018 /PRNewswire/ – Neovasc Inc. (“Neovasc” or the “Company”) (NASDAQ : NVCN )(TSX: NVCN), a leader in the development of minimally invasive transcatheter mitral valve replacement technologies and in the development of minimally invasive devices for the treatment of refractory angina, announced today (1) that it has received the decision of the […]
Neovasc Announces Second Quarter 2018 Financial Results
VANCOUVER, Aug. 8, 2018 /PRNewswire/ – Neovasc, Inc. (“Neovasc” or the “Company”) (NASDAQ: NVCN) (TSX: NVCN), a leader in the development of minimally invasive transcatheter mitral valve replacement technologies and in the development of minimally invasive devices for the treatment of refractory angina, today reported financial results for the second quarter ended June 30, […]
Neovasc Announces Collaboration and Licensing Agreement with Penn Medicine and the Gorman Cardiovascular Research Group
VANCOUVER, Aug. 3, 2018 /PRNewswire/ – Neovasc Inc. (“Neovasc” or the “Company”) (NASDAQ: NVCN) (TSX: NVCN), a leader in the development of minimally invasive transcatheter mitral valve replacement technologies, announced today that it has entered into a collaboration and licensing agreement relating to certain know-how developed by Penn Medicine and the Gorman Cardiovascular Research […]
Neovasc Announces Filing of “Administrative” Prospectus Supplement Relating to Expiry of Prior Shelf Prospectus / Registration Statement
VANCOUVER, July 16, 2018 /PRNewswire/ – Neovasc Inc. (“Neovasc” or the “Company”) (NASDAQ, TSX: NVCN) announced today that, in connection with its new final short form base shelf prospectus (the “Base Shelf Prospectus”) filed with securities regulatory authorities in the provinces of British Columbia, Alberta, Saskatchewan, Manitoba and Ontario (the “Provinces”) and its corresponding shelf registration statement on Form […]