ALISO VIEJO, Calif., Nov. 19, 2025 /PRNewswire/ — Okami Medical, a medical technology company pioneering novel vascular embolization solutions, today announced the successful close of an oversubscribed $45M financing led by new investor Gilde Healthcare with participation from existing…
Tag: Okami
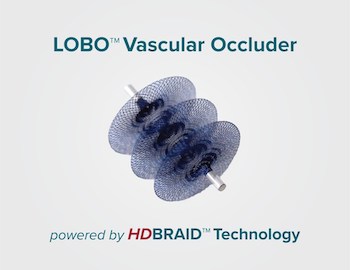
Okami Medical Announces FDA 510(k) Clearance of the LOBO-5 Vascular Occluder
ALISO VIEJO, Calif., Dec. 16, 2020 /PRNewswire/ — Okami Medical Inc., a medical device company, today announced the expansion of its LOBO™ Vascular Occlusion System product line with the U.S. FDA 510(k) clearance of the LOBO-5 Vascular Occluder. The LOBO (LOw-profile Braided Occluder) system is uniquely designed to provide interventional physicians with a single-device, […]
Okami Medical Announces Closing of $7.2M Series D Financing
ALISO VIEJO, Calif., June 24, 2020 /PRNewswire/ — Okami Medical Inc., announced today the close of a Series D financing totaling $7.2 million. The financing was led by U.S. Venture Partners and was joined by members of Okami’s board of directors and medical device industry veterans. The financing provides Okami, the second portfolio company of […]
Okami Medical Announces Major Milestones: FDA 510(k) Clearance And Key Patent For The LOBO Vascular Occluder
Microcatheter-delivered device designed to provide rapid and focal occlusion of a wide range of peripheral arterial targets ALISO VIEJO, Calif., Nov. 4, 2019 /PRNewswire/ — Okami Medical Inc., a medical device company, today announced 510(k) clearance by the U.S. Food and Drug Administration (FDA) and initial launch of the LOBOTM Vascular Occlusion System. […]