Key Highlights Osprey Medical has been selected as a Supplier Horizon Award recipient by Premier Inc. Osprey is one of nine suppliers to win the prestigious award, and is the only cardiovascular supplier to receive the award this year Award validates Osprey’s customer service and engagement, value creation and commitment […]
Tag: Osprey
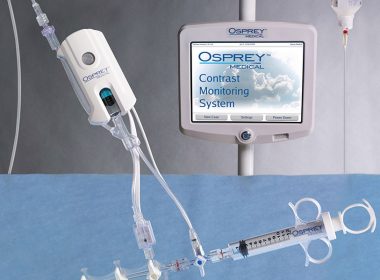
CE Marking Received for Core European Product, DyeVert Power XT
Key Highlights Osprey Medical has received European CE Marking approval for the 2nd generation DyeVert Power XT device Approval of DyeVert Power XT (for automatic injection) means the full coronary angiography market is now available to Osprey’s DyeVert franchise Device is expected to be a core product in the product portfolio […]
US Government to Transform Kidney Care
12 July, 2019 – Minnesota, United States and Melbourne, Australia – Osprey Medical (ASX: OSP) today announces its response to the US President signing an Executive Order aimed at transforming kidney care for more than 37 million Americans with some form of kidney disease. The initiative seeks to prevent kidney […]
Osprey to Deliver AKI Burden of Illness Data to Premier QUEST® Hospitals
June 13, 2019 – Minnesota, United States and Melbourne, Australia – Osprey Medical (ASX: OSP) today announces it is working with Premier Inc. to offer members of its QUEST® quality improvement collaborative hospital-level burden of illness reports on Acute Kidney Injury (AKI). These hospital-level reports originate from The AKI Burden […]
Osprey Appoints Mr Jim Surek as Vice President, Sales
March 4, 2019- Minnesota, United States and Melbourne, Australia – Osprey Medical (ASX: OSP) is pleased to announce the appointment of Mr Jim Surek as Vice President, Sales effective from March 1st. Mr Surek brings a wealth of experience and expertise to Osprey. He has over 25 years of senior […]
Osprey Study Chosen for “Best of Best” Presentation at Society for Cardiovascular Angiography and Interventions Conference
MINNESOTA, United States–(BUSINESS WIRE)– Osprey Medical Inc.(ASX:OSP) today announced a multi-center observational study for the DyeVert Plus™ System will feature at the Society for Cardiovascular Angiography and Interventions (SCAI) Scientific Session in April 2018. The abstract entitled: A Multi-Center Observational Study of the DyeVert Plus™ Contrast Reduction System summarizes a single-arm, multi-center observational […]