MINNEAPOLIS, June 15, 2022 /PRNewswire/ — VentureMed Group, Inc., a privately held medical device innovator in access management for arteriovenous (AV) fistulas and grafts and vessel preparation for interventional treatment of peripheral arterial disease (PAD) announced data presented at the Vascular Access Societies of the Americas (VASA) June 9-11, Charleston, South Carolina. Study Details and FindingsFLEX Vessel […]
Tag: PAD
According to THE SAGE GROUP, Gender is a Significant Factor in African American (AA) Peripheral Artery Disease (PAD) Disparities
BEAUFORT, S.C.–(BUSINESS WIRE)–Research published by THE SAGE GROUP examines PAD disparities in African Americans and the contributing factors, as well as quantifies PAD prevalence in AA. “While it is well known that African Americans have a higher prevalence of PAD, prior to this publication, there were no estimates of the […]
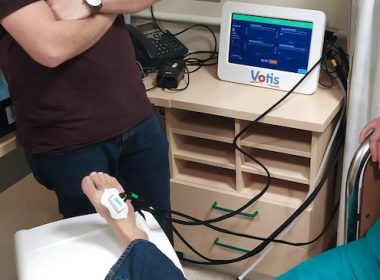
VOTIS SUBDERMAL IMAGING TECHNOLOGIES, LTD. ANNOUNCES ENROLLMENT OF FIRST HUMAN SUBJECT IN PILOT FEASIBILITY STUDY OF PEDCHECK™ AT HADASSAH MEDICAL CENTER IN JERUSALEM
JERUSALEM, May 2, 2022 /PRNewswire/ — VOTIS Subdermal Imaging Technologies Ltd. announced today that it has enrolled the first patient in the feasibility study of its PedCheckTM device at Hadassah Medical Center in Jerusalem, Israel. The pilot study, entitled “The Use of Hemodynamic Occlusive Vascular Response (HOVR™) technology to Screen for Peripheral Arterial Disease (PAD)”, is intended to […]
Respira Therapeutics Announces First Patient Dosed in Phase 2b VIPAH-PRN 2b Trial of RT234 in Patients with Pulmonary Arterial Hypertension (PAH)
First clinical trial to evaluate the safety & efficacy of RT234 on exercise parameters assessed by Cardiopulmonary Exercise Testing (CPET) PALO ALTO, Calif., April 5, 2022 /PRNewswire/ — Respira Therapeutics, Inc. a clinical-stage company developing inhaled therapeutics for cardiopulmonary diseases that improve patient outcomes through enhanced drug targeting to the small airways of […]
New Janssen Initiative Aims to Advance Equitable Care and Address Hidden Threat of Amputation Related to Peripheral Artery Disease (PAD)
Through Save Legs. Change Lives.™, Janssen seeks to elevate PAD research, collaboration, education and screening for communities placed at increased risk of cardiovascular disease TITUSVILLE, N.J., March 31, 2022 /PRNewswire/ — The Janssen Pharmaceutical Companies of Johnson & Johnson today announced the launch of Save Legs. Change Lives.™ Spot Peripheral Artery Disease Now, a multi-year […]
Ra Medical Systems Achieves Milestone of 100 Subjects Enrolled in its Pivotal Atherectomy Clinical Study
CARLSBAD, Calif.–(BUSINESS WIRE)–Ra Medical Systems, Inc. (NYSE American: RMED), a medical device company focusing on developing its excimer laser system to treat vascular diseases, announces the achievement of a milestone with the enrollment of 100 subjects in its pivotal clinical study to evaluate the safety and effectiveness of the DABRA […]
Regio Biosciences Enters into License Agreement with AstraZeneca for Phase 2a Asset in Peripheral Artery Disease (PAD)
Phase 2a clinical trial evaluating REG-101 for PAD expected to initiate second half of 2022 ROCKVILLE, Md.–(BUSINESS WIRE)–Regio Biosciences (Regio), a Hibiscus BioVentures company, announced today it has entered into an exclusive license agreement with AstraZeneca to further develop REG-101, a novel therapeutic acting on reverse cholesterol transport (RCT). Regio expects to initiate a […]
Cook Medical receives FDA breakthrough designation for new drug-eluting stent
Bloomington, Ind. — Cook Medical has received Breakthrough Device designation from the US Food and Drug Administration (FDA) on a new drug-eluting stent for below the knee (BTK). This new stent is designed to treat patients suffering from chronic limb-threatening ischemia (CLTI). “CLTI is a debilitating disease of growing prevalence around […]
Endologix Announces Completion of Enrollment in the TORUS 2 Study for PAD in SFA
The TORUS 2 study is a prospective, single-arm trial of 188 patients to evaluate safety and effectiveness of the TORUS Stent Graft System in the treatment of PAD in the SFA and proximal popliteal arteries IRVINE, Calif.–(BUSINESS WIRE)–Endologix LLC, a privately held global medical device company dedicated to improving patients’ […]
NEW RESEARCH FINDS CHALLENGES IN SYMPTOM RECOGNITION AND DIAGNOSTIC TESTING CAN IMPACT PATIENT SATISFACTION FOR PEOPLE WITH VASCULAR DISEASE
– One in four physicians surveyed feel the “lack of technology or equipment to make an accurate diagnosis of coronary artery disease and peripheral artery disease” is a key obstacle to an accurate diagnosis – Over a third of surveyed patients with Coronary Artery Disease (CAD) or Peripheral Artery Disease […]