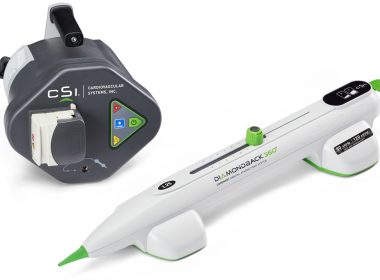
ST. PAUL, Minn.–(BUSINESS WIRE)–Cardiovascular Systems, Inc. (CSI®) (NASDAQ: CSII), a medical device company developing and commercializing innovative interventional treatment systems for patients with peripheral and coronary artery disease, announced today that the first patients in Germany have been treated with its Stealth 360® Peripheral Orbital Atherectomy System (OAS). The German cases […]
Tag: PAD
Eximo Medical Receives FDA Clearance for B-Laser™ Atherectomy System to Treat Peripheral Artery Disease (Including ISR)
REHOVOT, Israel–(BUSINESS WIRE)–Eximo Medical Ltd. announced today it has received 510(k) clearance from the U. S. Food & Drug Administration (FDA) for its B-Laser™ Atherectomy System for Peripheral Artery Disease (PAD). B-Laser™ is a transformative 355nm wavelength laser technology designed to address unmet clinical needs for treating multiple vascular indications. The specific indication cleared by the FDA is: […]
Avinger Announces 510(k) Filing of Pantheris Small Vessel Device
REDWOOD CITY, Calif., Aug. 30, 2018 (GLOBE NEWSWIRE) — Avinger, Inc. (NASDAQ:AVGR), a leading developer of innovative treatments for peripheral artery disease (PAD), today announced the Company submitted a new 510(k) application to the U.S Food & Drug Administration (FDA) for the Pantheris SV (Small Vessel) Lumivascular atherectomy system. Pantheris […]
Cardiovascular Systems Presents Liberty 360° Two-Year Outcomes at 2018 Amputation Prevention Symposium
ST. PAUL, Minn.–(BUSINESS WIRE)–Cardiovascular Systems, Inc. (CSI®) (NASDAQ: CSII), a medical device company developing and commercializing innovative interventional treatment systems for patients with peripheral and coronary artery disease, presented two-year outcomes from its LIBERTY 360° study in a late-breaking presentation at the 2018 Amputation Prevention Symposium (AMP) in Chicago. The […]
Semler Reports Second Quarter and First Half 2018 Financial Results
SAN JOSE, Calif., July 31, 2018 /PRNewswire/ — Semler Scientific, Inc. (OTCQB: SMLR), an emerging growth company that provides technology solutions to improve the clinical effectiveness and efficiency of healthcare providers, today reported financial results for the three and six months ended June 30, 2018. “We believe that the achievement of healthier clinical outcomes […]
Clinical Cardiology Publishes Study Demonstrating Simple Blood Test from Prevencio Accurately Detects Cardiovascular Disease
KIRKLAND, Wash.–(BUSINESS WIRE)–Prevencio, Inc., announces the publication of data that demonstrates a simple new blood test accurately diagnoses significant Peripheral Artery Disease (PAD), a circulatory problem in which plaque-narrowed arteries reduce blood flow to a patient’s limbs and kidneys. If left untreated, PAD can lead to clogged arteries and increase […]
Avinger Announces Successful Treatment of First Patients with Next-Generation Pantheris in Several Centers throughout the US
REDWOOD CITY, Calif., June 26, 2018 (GLOBE NEWSWIRE) — Avinger, Inc. (Nasdaq:AVGR), a leading developer of innovative treatments for peripheral artery disease (PAD), today announced that several physicians have successfully treated over 40 patients in the US across 13 sites with the next-generation Pantheris image-guided atherectomy system for the treatment […]
Mercator MedSystems Receives $11M in Series D Equity Financing Led by Shenzhen Salubris Pharmaceuticals Co.
EMERYVILLE, Calif., June 21, 2018 /PRNewswire/ — Mercator MedSystems, Inc., today announced it has received $11 million in Series D equity financing led by Shenzhen Salubris Pharmaceuticals Co. (Salubris), with participation from current Mercator investors and other undisclosed new investors. The new funding will further advance the clinical development of Mercator’s proprietary micro-infusion catheter systems […]
AngioSoma, Inc. Announces Selection of Regulatory Firm for Flagship Drug
HOUSTON, TX, June 18, 2018 (GLOBE NEWSWIRE) — AngioSoma, Inc. (SOAN) is excited to announce that we have identified a regulatory firm who can assist taking our patented flagship drug, Liprostin™, through the FDA approval process. The regulatory firm will begin with a presubmission meeting with the FDA biologic group […]
Micro Medical Solutions Marks First U.S. Implants of MicroStent for Peripheral Artery Disease
WILMINGTON, Mass.–(BUSINESS WIRE)–Micro Medical Solutions (MMS), an innovator in the field of microvascular interventions that aim to improve clinical outcomes and quality of life, announced a major milestone today in the development of its MicroStent technology, a vascular stent specifically designed to reduce below-the-knee amputations caused by critical limb ischemia […]