REDWOOD CITY, Calif., May 29, 2018 (GLOBE NEWSWIRE) — Avinger, Inc. (Nasdaq:AVGR), a leading developer of innovative treatments for peripheral artery disease (PAD), announced today that it will be presenting at the 8th Annual LD Micro Invitational at the Luxe Sunset Boulevard Hotel in Bel Air, California. The conference will be […]
Tag: PAD
XableCath Receives Second FDA Clearance for Its Peripheral Arterial Catheters
SALT LAKE CITY–(BUSINESS WIRE)–XableCath, Inc., innovators of products designed to aid in the treatment of peripheral artery disease (PAD), announced that its second catheter, XableCath™ abrasion tip support catheter, was cleared by the FDA. The XableCath blunt tip catheter received FDA clearance at the end of 2017. This clearance gives […]
FLEX Scoring Catheter Presented at 2018 Charing Cross International Symposium
TOLEDO, Ohio, May 7, 2018 /PRNewswire/ — VentureMed Group, Inc., a medical device company specializing in the development and commercialization of innovative and cost-effective devices for the endovascular treatment of peripheral arterial disease (PAD), presented a technical review of the FLEX Dynamic Scoring Catheter™ in the Innovations session of the conference, as […]
Vascugen Licenses Stem Cell Technology for Blood Vessel Regeneration Developed at IU School of Medicine
INDIANAPOLIS, May 1, 2018 /PRNewswire/ — Vascugen Inc. today announced that it has licensed a suite of intellectual property developed at the Indiana University (IU) School of Medicine related to blood vessel formation from adult stem cells. The regenerative medicine company is focused on finding therapies to repair tissues damaged by reduced blood flow due to disease […]
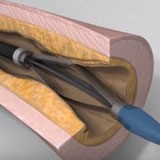
Avinger’s Next Generation Pantheris Featured in Live Case Transmission at Charing Cross International Symposium 2018
REDWOOD CITY, Calif., April 30, 2018 – Avinger, Inc. (AVGR), a leading developer of innovative treatments for peripheral artery disease (PAD), announced that its next generation Pantheris® Lumivascular atherectomy system with extended nosecone was featured in a live case transmission at Charing Cross Symposium (CX) 2018 held in London, England. Charing Cross […]
Medtronic IN.PACT(TM) Admiral(TM) Drug Coated Balloon Receives FDA Approval to Treat Long SFA Lesions
DUBLIN – April 23, 2018 – Medtronic plc (NYSE: MDT) today announced that it has received U.S. Food and Drug Administration (FDA) approval for the IN.PACT(TM) Admiral(TM) Drug-Coated Balloon (DCB) to treat long superficial femoral artery (SFA) lesions up to 360mm in patients with peripheral artery disease (PAD). Approval was based […]
Reflow Medical announces enrollment of its first patients in the Wing-IT IDE clinical trial
San Clemente, Calif., March 12, 2018 /PRNewswire/ — Reflow Medical Inc., a developer of innovative medical devices to combat cardiovascular disease, announced that the first patients have been enrolled in a prospective, multi-center, non-randomized study intended to evaluate the ability of the Reflow Wingman Catheters to cross chronic total occlusions […]
XableCath Granted United States Patent for its Peripheral Arterial Catheters
SALT LAKE CITY–(BUSINESS WIRE)– XableCath, Inc., innovators of products designed to aid in the treatment of peripheral artery disease (PAD), announced that it has been granted a second U.S. Patent, No. 9,826,995, covering its XableCath™ catheter. The patent specifically describes a catheter that controls tissue contact and has a rigid […]
Abbott and Surmodics Announce Agreement for Next-Generation Drug-Coated Balloon
ABBOTT PARK, Ill. and EDEN PRAIRIE, Minn., Feb. 27, 2018 /PRNewswire/ — Abbott (NYSE: ABT) and Surmodics (NASDAQ: SRDX) today announced that the companies have entered into an agreement whereby Abbott will have exclusive worldwide commercialization rights for Surmodics’ SurVeil® drug-coated balloon to treat the superficial femoral artery, which is currently being evaluated in a U.S. pivotal […]
PQ Bypass Announces First Patient Treated in Landmark DETOUR II Trial Evaluating New Treatment Approach for Clogged Leg Arteries
SUNNYVALE, Calif.–(BUSINESS WIRE)– PQ Bypass, a clinical-stage medical technology company that has developed the DETOUR procedure, a novel treatment for long (>15 cm) superficial femoral artery (SFA) blockages, announced enrollment of the first patients in the pivotal DETOUR II Trial in the United States. The trial is a prospective, single-arm clinical […]