REDWOOD CITY, Calif., Dec. 20, 2017 (GLOBE NEWSWIRE) — Avinger, Inc. (NASDAQ:AVGR), a leading developer of innovative treatments for peripheral artery disease (PAD), today announced Conformité Européenne (CE) Marking approval for its next generation Pantheris Lumivascular atherectomy system, the first-ever image-guided atherectomy device for the treatment of PAD. The novel […]
Tag: PAD
PQ Bypass Receives IDE Approval to Initiate Study of First-of-its-Kind Procedure for Patients Suffering From Peripheral Artery Disease
SUNNYVALE, Calif.–(BUSINESS WIRE)–PQ Bypass, Inc., today announced it has received conditional approval of its investigational device exemption (IDE) from the U.S. Food and Drug Administration (FDA) to initiate the pivotal DETOUR II clinical trial. As the first-ever pivotal trial for percutaneous femoropopliteal bypass, DETOUR II will evaluate the safety and […]
Janssen Submits Supplemental NDA to FDA Seeking New Indications for XARELTO (rivaroxaban) for Patients With Chronic Coronary and/or Peripheral Artery Disease (CAD/PAD)
RARITAN, N.J., Dec. 11, 2017 /PRNewswire/ — Janssen Research & Development today announced it has submitted a supplemental New Drug Application (sNDA) to the U.S. Food and Drug Administration (FDA) for two new XARELTO® (rivaroxaban) vascular indications: reducing the risk of major cardiovascular (CV) events such as CV death, heart attack or stroke […]
PVD Patients Boomeranging at Higher Rates After Intervention, Study Shows
By Ken Dropiewski, Prime-Core Executive Search (ken@prime-core.com) As we progress to value-based healthcare system, it’s more important than ever to keep readmissions to a minimum. A recent study, though, found that more than 17 percent of patients undergoing peripheral revascularization for PVD were readmitted within 30 days of having the procedure.The unplanned […]
Cagent Vascular Wins CE Mark for Serranator, a Next Generation Device for Vessel Dilatation in PAD Interventions
WAYNE, Pa.–(BUSINESS WIRE)– Cagent Vascular, a developer of next generation technology for vessel dilatation in cardiovascular disease interventions, announces the issuance of its CE Marking for the Serranator® PTA Serration Balloon Catheter. Cagent Vascular has also achieved its ISO 13485 Certification. The Serranator® is one of a family of peripheral artery disease (PAD) […]
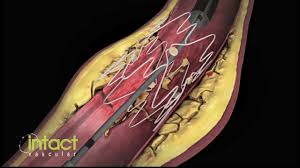
Positive Twenty-Four Month Tack Optimized Balloon Angioplasty (“TOBA”) Single Center Results Presented at VEITHsymposium™ Conference
WAYNE, Pa.–(BUSINESS WIRE)–Intact Vascular, Inc., a developer of medical devices for minimally-invasive peripheral vascular procedures, today announced that positive single center twenty-four month results from its Tack Optimized Balloon Angioplasty (TOBA) clinical study were presented at the VEITHsymposium™ 2017 conference by Dr. Christian Wissgott, Assistant Director, at Westküstenklinikum Heide in Heide, Germany. The TOBA study enrolled […]
Aziyo Presents Positive Initial Data From Clinical Study of Biologic Extracellular Matrix Scaffold for Femoral Arterial Reconstruction
SILVER SPRING, Md.–(BUSINESS WIRE)– Aziyo Biologics, a fully integrated regenerative medicine company, today announced positive results from PERFORM, a prospective, post-market study conducted in patients undergoing femoral arterial reconstruction using the company’s biologic extracellular matrix (ECM) scaffold. There were no device-related adverse events or infections and primary patency was maintained in 97.6% of […]
Postmarket Surveillance Study Confirms Benefits of Cook Medical’s Zilver® PTX® Drug Eluting Stent at 12 Months in Japanese Patients
LAS VEGAS–(BUSINESS WIRE)–One-year post-market surveillance (PMS) study data from Japanese patients who received the Zilver® PTX® drug-eluting stent confirmed the benefits of the device at 12 months, researchers announced today. Japanese researchers presented the PMS study results at themeeting in Las Vegas. This was the first time the study has been presented […]
Symic Bio Announces Results from the Phase 1/2 SHIELD Trial of SB-030 in Peripheral Vascular Disease
SAN FRANCISCO, Nov. 1, 2017 /PRNewswire/ — Symic Bio, a biopharmaceutical company developing novel matrix-targeting biotherapeutics, today announced results from the Phase 1/2 SHIELD trial evaluating SB-030, a locally administered therapeutic, in patients with peripheral vascular disease undergoing angioplasty. In this first-in-human, prospective, randomized, single-blind controlled study of 67 patients, SB-030 demonstrated a […]
Surmodics Announces First Patient Enrolled in TRANSCEND Pivotal Clinical Trial for SurVeil® Drug-Coated Balloon
EDEN PRAIRIE, Minn.–(BUSINESS WIRE)–Surmodics, Inc. (Nasdaq: SRDX), a leading provider of medical device and in vitro diagnostic technologies, today announced enrollment of the first patient in TRANSCEND, the pivotal clinical trial for the SurVeil® drug-coated balloon (DCB). The randomized trial will evaluate the SurVeil DCB for treatment for peripheral artery disease (PAD) in […]