LOS ANGELES, Oct. 03, 2017 (GLOBE NEWSWIRE) — Advanced Bifurcation Systems (“ABS” or the “Company”), a clinical stage medical device company developing an innovative stenting platform which overcomes the limitations of current approaches for the treatment of bifurcation lesions in coronary angioplasties, today announced that the Company is planning to […]
Tag: PCI
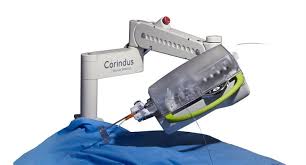
Corindus Announces Opening of First International Robotic Training Center
WALTHAM, Mass.–(BUSINESS WIRE)–Corindus Vascular Robotics, Inc. [NYSE American: CVRS], a leading developer of precision vascular robotics, announced today the opening of the first international CorPath GRX Robotic Center in Tokyo, Japan. Earlier this year, Corindus partnered with Japan Medicalnext Co., Ltd., a wholly-owned entity of MC Healthcare, Inc. (subsidiary of […]
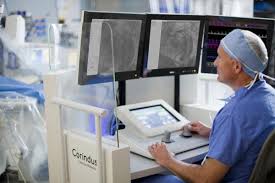
Corindus Announces First Patient Enrolled in PRECISION GRX Registry
WALTHAM, Mass.–(BUSINESS WIRE)–Corindus Vascular Robotics, Inc. [NYSE American: CVRS], a leading developer of precision vascular robotics, today announced first patient enrollment in the PRECISION GRX Registry, a post-market study to continue market surveillance of the Company’s second generation CorPath GRX System. The PRECISION GRX Registry will include up to 25 sites […]
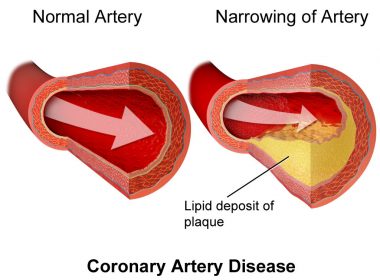
What is the Significance of Lipid-rich Plaque and Should it Influence Targets for PCI?
By Ken Dropiewski Lipid-rich plaque in non-culprit lesions has long been associated with subsequent cardiac events. Unlike culprit lesions, known to be the cause of AMI, non-culprit lesions including those with a core of lipid-rich plaque are often left untreated during an intervention. The fact that these lesions may lead […]
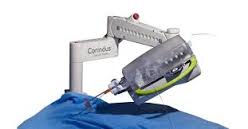
Corindus Vascular Robotics Announces Live Broadcast Of Robotic-Assisted PCI Using CorPath GRX To TCT Asia-Pacific Summit In South Korea
Procedure transmitted live from Fu Wai Hospital to over 4,000 attendees at 22nd annual TCT Asia-Pacific Cardiovascular Summit WALTHAM, Mass.–(BUSINESS WIRE)–Corindus Vascular Robotics, Inc. [NYSE MKT: CVRS], a leading developer of precision vascular robotics, announced today that Fu Wai Hospital in China performed and broadcast live a PCI procedure using […]
New Study Finds Abiomed Impella® Heart Pump Reduces Injury to Kidneys During High-Risk Percutaneous Coronary Intervention
DANVERS, Mass., March 09, 2017 (GLOBE NEWSWIRE) — A new study published in Circulation Research finds use of hemodynamic support with Impella® 2.5 heart pump during high-risk percutaneous coronary intervention (HRPCI) can reduce the risk of acute kidney injury (AKI) even when those patients had preexisting kidney disease1 and low […]
Teleflex (TFX) Announces 510(k) Clearance and U.S. Launch of Spectre™ Guidewire
View source version on businesswire.com: http://www.businesswire.com/news/home/20170314005152/en/ Teleflex Incorporated (TFX), a leading global provider of medical technologies for critical care and surgery, has announced 510(k) clearance by the Food and Drug Administration and U.S. commercial launch of the Spectre Guidewire. The Spectre Guidewire is engineered with a smooth stainless steel-to-nitinol dual-core […]