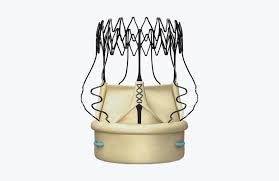
Perceval Sutureless Aortic Heart Valve Received Approval in China to Treat Patients with Aortic Valve Disease LONDON–(BUSINESS WIRE)–CORCYM, the medical device company dedicated to providing patients and cardiac surgeons with the best solutions to fight structural heart disease, announces today the first Perceval implant in China, after the recent approval from the […]
Tag: Perceval
New Data on LivaNova Perceval Sutureless Aortic Valve Show Consistent Outcomes, Lower Procedure Times Compared to Sutured Valves
PERSIST-AVR study results presented at American Association for Thoracic Surgery Annual Meeting LONDON–(BUSINESS WIRE)–LivaNova PLC (NASDAQ:LIVN), a market-leading medical technology and innovation company, presented the first data from the Perceval® Sutureless Implant Versus Standard-Aortic Valve Replacement (PERSIST-AVR) clinical study at the 100th Annual Meeting of the American Association of Thoracic Surgery (AATS). The study demonstrated that the […]
LivaNova’s Perceval Sutureless Aortic Heart Valve Receives National Reimbursement in Japan to Treat Patients with Aortic Valve Disease
LONDON–(BUSINESS WIRE)–LivaNova PLC (NASDAQ:LIVN), a market-leading medical technology company, today announced that Japan’s Ministry of Health, Labour and Welfare granted national reimbursement for the Company’s Perceval® sutureless aortic heart valve to treat aortic valve disease. By adding Perceval into Japan’s health insurance system, physicians and patients have greater access to this […]
New Studies in the U.S. and Europe Demonstrate LivaNova Perceval Valve’s Strong Performance and Economic Advantage in Aortic Disease Treatment
LONDON–(BUSINESS WIRE)–LivaNova PLC (NASDAQ:LIVN), a market-leading medical technology company, today announced the publication of three separate studies highlighting the unique performance of its sutureless aortic valve, Perceval®. The results from the Perceval Aortic Heart Valve Study in North America, performed under a U.S. Food and Drug Administration (FDA) Investigational Device […]
LivaNova’s Perceval Sutureless Aortic Heart Valve Approved in Japan
LONDON–(BUSINESS WIRE)–LivaNova PLC (NASDAQ:LIVN) (“LivaNova” or the “Company”), a market-leading medical technology company, today announced that Japan’s Ministry of Health, Labour and Welfare has approved the Company’s Perceval® sutureless aortic heart valve to treat aortic valve disease. “With this approval for Perceval, an innovative and trusted valve platform, we are able […]