December 15, 2025
Tag: Philips
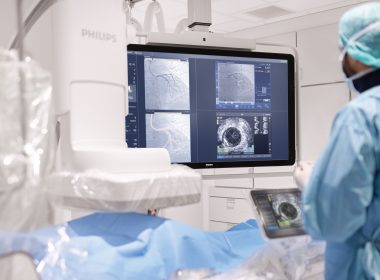
Philips and Cortechs.ai extend partnership to advance quantitative neuroimaging and strengthen Philips’ leadership in precision diagnostics in neurology
November 19, 2025Amsterdam, the Netherlands – Royal Philips (NYSE: PHG, AEX: PHIA), a global leader in health technology, today announced an extended partnership with Cortechs.ai, a pioneer in quantitative neuroimaging solutions. Together, the companies will integrate Cortechs.ai’s advanced AI-enabled neuroimaging analytics directly into Philips’ MR systems [1], empowering clinicians with faster, more objective, and reproducible insights into brain health. The collaboration strengthens Philips’ leadership in precision neuro diagnostics, combining Philips’ next-generation MR technologies with Cortechs.ai’s AI-driven quantitative neuro imaging post processing software [1] to transform how neurological diseases are detected, monitored, and managed.Combining complementary expertise for smarter brain imagingUp to 25% [2] of all MR procedures are brain scans, and radiology departments face growing demand amid increasing staff shortages. At the same time, the number of patients with neurological conditions such as Alzheimer’s disease, multiple sclerosis (MS), and brain tumors continue to rise globally. Current MR interpretation of neurodegenerative diseases or neuro oncology cases depends heavily on visual assessment, introducing variability and requiring significant expertise and time in an already overloaded system.Through this extended partnership, Philips and Cortechs.ai aim to deliver more standardized and data-driven imaging. By integrating quantitative analysis tools such as brain volumetrics, lesion quantification, and tumor tracking directly into the Philips MR imaging workflow [1], clinicians gain objective, numerical data alongside images – enabling faster, more consistent, and confident diagnoses.Empowering clinicians with precision and speedThe integration of Cortechs.ai’s NeuroQuant® solutions for Alzheimer’s, MS and brain tumors within Philips’ Smart Reading environment – a cloud-based AI platform that combines imaging, reading, and reporting directly on the MR systems [1] – marks a major step toward precision neuroimaging.With this integration, clinicians can automatically receive AI-generated quantitative reports within their standard MR workflow on the MR system or PACS, without adding extra steps for technologists. The zero-click process ensures quality-checked data and standardized outputs, helping radiologists interpret brain scans more objectively, faster and with greater confidence.“By extending our partnership with Cortechs.ai, we are accelerating the transition to fully quantitative, AI-powered neuroimaging,” said Ioannis Panagiotelis, PhD, Business Leader, MRI, Philips. “This integration brings precision, reproducibility, and efficiency to neurological MR, helping clinicians make faster, more confident decisions for patients living with complex brain conditions.”Driving productivity and improving care through AIPhilips is driving productivity and improving care through deep AI integration that tackles some of healthcare’s most pressing challenges – from workforce shortages to the growing demand for advanced diagnostics. By automatically generating quantitative reports within existing workflows, AI helps radiologists save time, improve consistency, and boost throughput. Objective numerical biomarkers enhance diagnostic accuracy, enabling more reproducible assessments and confident comparisons across time and sites. And by minimizing manual post-processing through automated, quality-controlled workflows, technologists can focus more on what matters most – delivering a better, more personalized patient experience.“Cortechs.ai’s mission has always been to empower clinicians with actionable, quantitative insights,” said Kyle Frye, Chief Executive Officer, Cortechs.ai. “By combining our advanced neuro analytics with Philips’ leading MR platform and AI infrastructure, we’re making it easier to personalize brain care and track neurological changes with precision.”Setting a new standard in precision neuro diagnosticsCortechs.ai’s NeuroQuant® solutions are already available on Philips Advanced Visualization Workspace. With this extended partnership, Philips reinforces its commitment to advancing the field of neurology – empowering healthcare providers to deliver better care for more people, in less time, with greater confidence.
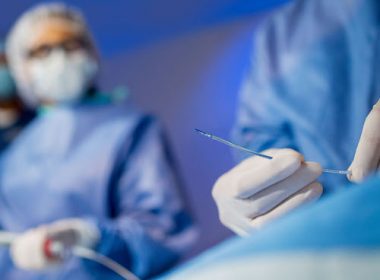
Philips brings AI into the procedure room to assist doctors during heart valve repair
November 17, 2025Innovation uses AI to track and visualize tiny repair devices* through the beating heart, helping clinicians navigate in 3D with enhanced clarity and confidence**Amsterdam, the Netherlands – Royal Philips (NYSE: PHG, AEX: PHIA), a global leader in health technology, today introduced DeviceGuide, an AI-powered device tracking* solution that assists physicians during one of interventional cardiology’s most technically demanding procedures: repairing leaking heart valves through a minimally invasive approach. Built on Philips’ EchoNavigator platform, this software brings AI directly into the procedure room, translating complex imaging into intuitive, real-time visual guidance that helps clinicians navigate the beating heart with greater clarity and confidence. It will be previewed at London Valves 2025 (November 16-18), one of the world’s leading meetings for structural heart specialists.“With DeviceGuide, we’re bringing AI into the heart of the procedure room, and into the heart itself,” said Dr. Atul Gupta, Chief Medical Officer, Diagnosis & Treatment at Philips. “This is Philips’ first AI assisting physicians in real time to visualize and guide heart valve treatment devices* as they navigate the beating heart. It’s helping doctors in the moment as they are helping their patients with structural heart disease.”Minimally invasive treatment of the mitral valveA leaking heart valve, known as mitral valve regurgitation, is a condition in which blood leaks backward through the heart’s mitral valve. It affects more than 35 million [1] adults worldwide, leaving many short of breath, fatigued, and struggling with everyday activities like climbing stairs or walking short distances. Many also live with anxiety or fear, knowing their heart isn’t pumping efficiently. If left untreated, severe cases can lead to heart failure and other serious complications.For patients who are too frail for open-heart surgery, minimally invasive transcatheter repair techniques such as mitral transcatheter edge-to-edge repair (M-TEER) offer a vital treatment option. During these procedures, physicians repair a leaking valve through a tiny incision in the top of the leg in the groin area, guiding long, flexible instruments through the blood veins and navigating a miniature repair device into the beating heart.Clinicians must view and interpret both X-ray and ultrasound images on multiple screens, coordinate movements between two operators, and make precise adjustments to grasp the moving valve leaflets, position the repair device, and confirm the result in real time. The process demands accuracy, coordination, and experience from the team. This is where DeviceGuide can help with its 3D navigation support.How AI is helpingDeviceGuide uses an AI algorithm to automatically track the tiny repair device as it moves through the beating heart, intelligently combining live echo and X-ray images. It creates a virtual 3D model of the treatment device in real time, superimposed on the live images of the beating heart. This allows clinicians to see where the device is and in which direction it is pointing, providing a clear view of the procedure and potentially enabling them to navigate the device more easily to effectively seal the leak.“The AI software serves as an assistive tool, with the physician always remaining in control. This isn’t about replacing expertise – it’s about amplifying it,” added Dr. Atul Gupta. “By embedding AI into the procedure, DeviceGuide gives physicians an extra pair of eyes, effectively bionic vision, helping them treat more patients safely and confidently.”Collaboration with Edwards LifesciencesThis innovation was developed in close collaboration with Edwards Lifesciences, the manufacturer of these repair devices. The solution combines Philips’ expertise in medical imaging and AI technologies with Edwards’ global leadership in structural heart innovation. Together, the companies have reimagined key parts of the mitral TEER procedural workflow.“DeviceGuide demonstrates the impact of combining leading imaging and therapy expertise to develop solutions designed around the procedural workflow, a model that will shape the future of AI-enabled image-guided structural interventions,” said Mark Stoffels, Business Leader, Image Guided Therapy Systems.This solution illustrates how Philips’ imaging and AI expertise can merge with the insights of leading therapy companies to innovate procedural workflows. This kind of collaboration is a model for developing the next generation of AI-enabled, image-guided innovations, centered on the needs of clinicians and their patients.Read more on how Philips DeviceGuide works here.
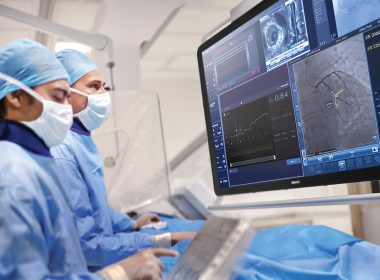
Late-breaking iMODERN findings presented at TCT 2025 and published in the New England Journal of Medicine highlight new evidence to guide treatment choices for heart attack patients
Philips iFR technology
October 29, 2025 3-Year data from the largest global trial to date using Class I, Level A iFR in heart attack patients shows treating additional stenoses in the same procedure showed no significant difference in major outcomes compared with waiting for follow-upAdditional results from the ILIAS ANOCA study highlight the sustained benefits of physiology-guided assessment and tailored medical therapy for patients with angina and no obstructive coronary arteries Amsterdam, the Netherlands – Royal Philips (NYSE: PHG, AEX: PHIA), a global leader in health technology, today announced late-breaking results from the iMODERN trial (Instantaneous wave-free Ratio Guided Multivessel Revascularisation During PCI for Acute Myocardial Infarction) at the Transcatheter Cardiovascular Therapeutics (TCT) 2025 conference, the world’s leading meeting for interventional cardiology. The study compared immediate versus delayed treatment of additional narrowed arteries in heart attack patients to determine whether treating all blockages in one procedure is as safe and effective as waiting for a follow-up procedure. The study compared immediate versus deferred treatment of additional narrowed arteries in heart attack patients to determine whether treating all blockages in a single procedure is superior to waiting for a follow-up procedure – a key question, since many patients have multiple diseased arteries and the optimal timing for complete treatment remains uncertain. In today’s practice, when patients suffer a serious type of heart attack (STEMI), cardiologists urgently open the blocked artery causing the attack. But many of these patients also have disease in other arteries. Often these additional narrowed arteries are not treated right away, either due to time restraints, patient stability, resource constraints or because they stay unnoticed. They may be treated later in a separate hospital stay or not at all, leaving uncertainty about the best timing and approach. The new findings show that patients can safely have additional arteries treated immediately during the first procedure to treat the acute event, rather than during a later second intervention. By confirming the safety of extending Class I, Level A-recommended iFR to non-stable patients, the results offer physicians the opportunity to complete treatment in one session, without compromising long-term outcomes. The iMODERN trial is the largest study to date testing iFR* in the acute heart attack setting, expanding the evidence base for a tool already strongly recommended (Class I, Level A) in stable patients. The findings are published in The New England Journal of Medicine (NEJM), underscoring its global scientific significance and impact on cardiology practice. “These results address one of the longest-standing questions in interventional cardiology,” said Prof. Niels van Royen, co-principal investigator, Radboud University Medical Center, The Netherlands. “Measuring and eventually treating additional arteries can be performed during the first procedure or during a staged procedure. That means cardiologists can feel confident offering patients a complete solution in one sitting when it’s appropriate.” The iMODERN study enrolled 1,146 patients across 41 hospitals in 14 countries directly addressed this question. Patients were randomly assigned to one of two treatment strategies: either immediate physiology-guided treatment of additional narrowed arteries during the first procedure using instantaneous wave-free ratio (iFR), or staged treatment guided by cardiac Magnetic Resonance Imaging (MRI) carried out within four days to six weeks after the heart attack. The study’s main endpoint combined three outcomes: death, another heart attack, or hospitalization for heart failure over three years. After three years of follow-up, the trial found no significant difference in major outcomes – including death, repeat heart attack, or hospitalization for heart failure – between the two approaches. By confirming that both approaches are backed by solid evidence, the trial offers patients more certainty and more personalized care. “Flexibility is critical in real-world practice,” added Prof. Dr. Robin Nijveldt, co-principal investigator, at Radboud University Medical Center, in the Netherlands. “Some patients may benefit from immediate treatment, while others are better served by waiting. iMODERN is a pragmatic study that shows that an immediate intervention is not necessarily better than waiting, if patients are offered a CMR to evaluate the need for a second intervention, giving physicians the evidence they need to tailor decisions to each patient.” “These results complement current international guideline recommendations (Class I recommendation, Level A evidence) for complete revascularization in STEMI,” said Dr. Darshan Doshi, practicing interventional cardiologist and Head of Medical & Clinical at Philips Image-Guided Therapy Devices. “By integrating physiological assessment, iMODERN’s evidence demonstrates that cardiologists can follow these findings for full revascularization while also tailoring treatment to each vessel’s true ischemic relevance.” Complementary evidence from ILIAS ANOCA In related findings presented at the same conference, the ILIAS ANOCA (Inclusive Invasive Physiological Assessment in Angina Syndromes – Angina with No Obstructive Coronary Artery Disease) study further demonstrated the value of physiology-guided decision-making — this time in patients with angina and no obstructive coronary arteries (ANOCA). The ILIAS ANOCA study evaluated the impact of coronary function testing (CFT) in patients whose coronary arteries appear unobstructed on angiography but who continue to experience angina. Conducted across five cardiac centers in the Netherlands and Germany (n=153), the investigator-initiated, randomized, blinded-arm controlled trial compared standard care with CFT-guided medical therapy using Philips Doppler FloWire and FloMap systems. The study found that ad-hoc CFT followed by tailored therapy significantly improved patient-reported angina symptoms and quality of life at six months, with a mean 9.4-point gain in the Seattle Angina Questionnaire summary score (95% CI 3.9–14.9; p=0.001). There were no procedure-related complications or major adverse cardiac events. These results confirm and extend the earlier CorMiCA findings, supporting CFT as a Class I, Level A recommendation in ANOCA patients and demonstrating the potential of Philips Doppler technology to guide individualized treatment strategies safely and effectively. The 12-month results presented on October 26th demonstrate the sustained benefits of CFT-guided care. iFR and MRI technologyPhilips physiology solutions – including its instantaneous wave-free ratio (iFR) pressure wires and software – were used to guide immediate treatment decisions in the trial. Philips also provides advanced cardiac MRI technology, which guided the delayed strategy. By enabling both the invasive and non-invasive approaches evaluated in iMODERN, Philips supported the generation of robust evidence to help guide clinical practice worldwide. * iFR (instantaneous wave-free ratio) is a minimally invasive way to measure blood pressure through the coronary arteries, helping physicians decide which blockages require stenting. For further information, please contact: Joost MalthaPhilips Global External RelationsTel.: +31 6 1055816E-mail: joost.maltha@philips.com About Royal Philips Royal Philips (NYSE: PHG, AEX: PHIA) is a leading health technology company focused on improving people’s health and well-being through meaningful innovation. Philips’ patient- and people-centric innovation leverages advanced technology and deep clinical and consumer insights to deliver personal health solutions for consumers and professional health solutions for healthcare providers and their patients in the hospital and the home.Headquartered in the Netherlands, the company is a leader in diagnostic imaging, ultrasound, image-guided therapy, monitoring and enterprise informatics, as well as in personal health. Philips generated 2024 sales of EUR 18 billion and employs approximately 67,800 employees with sales and services in more than 100 countries. News about Philips can be found at www.philips.com/newscenter.
Attachment
Philips iFR technology
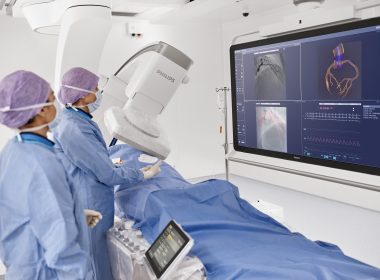
Philips introduces industry-first cath lab integration that automatically synchronizes pre-operative CT with C-arm movement, paving the way for CT-guided PCI
Philips Follow C-arm capability
Follow C-arm
October 27, 2025 New integration of Philips Advanced Visualization Workspace* with the Azurion image-guided therapy platform automatically synchronizes CT images with C-arm movement, supporting workflow efficiency and additional anatomical insights in PCI procedures1 San Francisco, USA and Amsterdam, The Netherlands – At the annual Transcatheter Cardiovascular Therapeutics (TCT 2025) meeting, Royal Philips (NYSE: PHG, AEX: PHIA), a global leader in health technology, today introduced an industry-first innovation that integrates pre-operative CT data directly into the cath lab workflow. This new capability, available through the integration of Philips’ Advanced Visualization Workspace (AVW) with the Azurion image-guided therapy system, marks a first step towards CT-guided percutaneous coronary intervention (PCI), a minimally invasive procedure to open narrowed coronary arteries and restore blood flow to the heart.The new capability, Follow C-arm, automatically synchronizes the 3D reconstruction of coronary arteries with the movement of the Azurion C-arm. As the C-arm angulation changes, the CT volume rotates in real time to match, giving interventionalists the 3D anatomical view without manual interaction. This seamless connection helps clinicians combine the detailed insights of CT imaging with the flexibility of live X-ray guidance inside the cath lab. The combined AVW–Azurion approach aims to provide enhanced anatomical insights to guide complex PCI procedures, publications have shown that leveraging CCTA may lead to reduction in contrast medium use and radiation dose during interventions.1Supporting the shift towards CT-guided PCICoronary computed tomography angiography (CCTA) is increasingly used in global clinical guidelines as a first-line tool for the diagnosis and planning of coronary artery disease. With more patients now arriving at the cath lab with prior CT scans, physicians are seeking ways to incorporate this information into their interventional workflows. By integrating CT data directly into Azurion, Philips is helping interventionalists expand the use of CT beyond diagnosis and planning, supporting a future in which CT-guided PCI becomes standard practice.“By bringing pre-operative CT into the cath lab and linking it directly to the movement of the C-arm, Philips is delivering an industry-first that helps interventionalists prepare for and execute PCI procedures with greater confidence,” said Mark Stoffels, Business Leader Image-Guided Therapy Systems at Philips. “This seamless integration is a significant step towards CT-guided PCI, aligning with our commitment to improving workflow efficiency and advancing patient care in interventional cardiology.”The launch builds on the global success of Philips’ Azurion image-guided therapy platform, the world’s leading system designed for seamless integration of advanced applications. Since its introduction in 2017, Azurion has been used to treat more than 6.4 million patients annually in over 80 countries, helping physicians perform minimally invasive procedures with greater confidence and efficiency.For more information about Philips’ presence at TCT, please visit the Philips TCT 2025 landing page. For further information, please contact:Joost MalthaPhilips Global External RelationsTel.: +31 6 1055816E-mail: joost.maltha@philips.com About Royal Philips Royal Philips (NYSE: PHG, AEX: PHIA) is a leading health technology company focused on improving people’s health and well-being through meaningful innovation. Philips’ patient- and people-centric innovation leverages advanced technology and deep clinical and consumer insights to deliver personal health solutions for consumers and professional health solutions for healthcare providers and their patients in the hospital and the home.Headquartered in the Netherlands, the company is a leader in diagnostic imaging, ultrasound, image-guided therapy, monitoring and enterprise informatics, as well as in personal health. Philips generated 2024 sales of EUR 18 billion and employs approximately 67,800 employees with sales and services in more than 100 countries. News about Philips can be found at www.philips.com/newscenter. Disclaimer: * The Follow C-arm capability as part of Philips Advanced Visualization Workspace may not be available in all markets. Please contact your Philips representative for more details.Reference: 1. J Cardiovasc Comput Tomogr. 2025 May-Jun;19(3):277-290.
Attachments
Philips Follow C-arm capability
Follow C-arm
Philips joins Optum Healthcare’s network as a preferred provider in the USA
Amsterdam, the Netherlands – Royal Philips (NYSE: PHG, AEX: PHIA), a global leader in health technology, today announced that it has entered a national partnership in the USA with Optum Healthcare. The inclusion of Philips’ Mobile Cardiac Telemetry (MCOT) and Philips Extended Holter (ePatch) in the network is designed to enable earlier detection of […]
Philips launches smart telemetry platform for cardiac monitoring that provides continuous, enterprise-wide connectivity beyond the bedside
September 4, 2025
Philips launches Transcend Plus for EPIQ CVx and Affiniti CVx, delivering breakthrough image quality and FDA-cleared AI enhancements in cardiovascular ultrasound
August 27, 2025
Philips Announces Plan for More Than USD 150 Million of New Investment in Manufacturing and R&D in the U.S. to Expand Production of AI-powered Health Technology Innovations
Global health technology leader, which serves 90% of U.S. hospitals, builds on top of existing U.S. investments to support local manufacturing and more than USD 900 million of annual R&D investment in the U.S. Philips EPIQ Elite Ultrasound CAMBRIDGE, Mass.–(BUSINESS WIRE)–Philips, a global leader in health technology, today announced a plan […]
Philips announces collaboration with Epic to enhance ambulatory cardiac monitoring
July 24, 2025