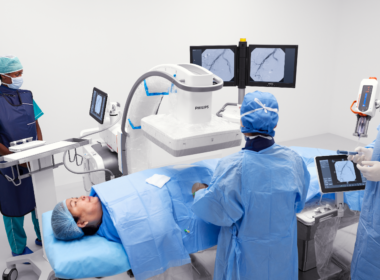
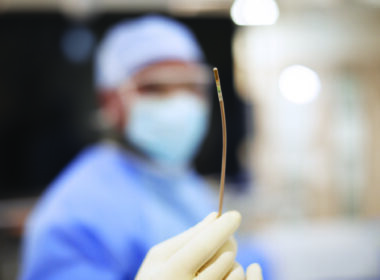
Amsterdam, the Netherlands – Royal Philips (NYSE: PHG, AEX: PHIA), a global leader in health technology, today announced the introduction of the 160cm FDA approved version of its unique LumiGuide endovascular navigation wire. This enhanced LumiGuide guidewire, which utilizes the company’s breakthrough Fiber Optic RealShape (FORS) technology, was used for the first time on a patient by Professor and vascular surgeon Carlos Timaran, MD, an internationally recognized expert in advanced endovascular techniques. He used the technology during a complex aortic aneurism repair operation, marking the 1000th patient treated milestone using FORS technology since first clinical use of the technology in 2020. The introduction of a longer FORS-enabled LumiGuide Navigation Wire enables US clinicians to visualize a broader range of catheters and expands usage of this technology to patients in America treated in centers equipped with LumiGuide.
Tag: Philips
Carilion Clinic further expands quality cardiac care access with latest Philips innovations at new regional cardiovascular institute in Virginia, US
August 20, 2024
Bon Secours Mercy Health and Philips sign multi-year strategic collaboration
July 17, 2024 Patient monitoring platform to deliver tools and time to help patients across 40+ care sites Amsterdam, the Netherlands, and Cincinnati, Ohio – Royal Philips (NYSE: PHG, AEX: PHIA), a global leader in health technology and Bon Secours Mercy Health (BSMH), one of the nation’s largest Catholic health systems, today announced a multi-year strategic collaboration for patient monitoring. Putting BSMH clinicians in control, the collaboration will reduce the digital burden on staff and give them more time to spend with patients. The partnership provides access to the latest Philips monitoring innovations, including a scalable patient monitoring platform that integrates patient data and provides vital insights. It will standardize patient monitoring for BSMH’s 49 hospitals, reducing costs through a predictable payment model and enabling further reinvestment in innovation. “This collaboration is part of our commitment to drive improved healthcare quality while reducing costs and addressing healthcare issues facing entire communities,” said Jodi Pahl, Chief Nursing Officer for workforce experience and nursing outcomes, Bon Secours Mercy Health. “This 10-year journey will bring innovations that will transform care delivery.” BSMH provides patients care more than 11 million times annually through its network of more than 1,200 care sites, 60,000 associates and 49 hospitals serving communities in Florida, Kentucky, Maryland, New York, Ohio, South Carolina and Virginia, as well as Ireland. “With implementation occurring over the next three years, followed by seven years of maintenance and updates, we’re positioned to be at the leading edge of technology,” said Pahl. “This uplifts our Mission to improve the health and well-being of our communities and supports our commitments to innovation and stewardship.” Julia Strandberg, Chief Business Leader, Connected Care at Philips said: “This partnership is a testament to BSMH’s focus on strong clinical engagement and our combined commitment to improving the patient and staff experience. As BSMH’s clinical technology partner, we’re leaning in to understand their needs and apply innovative technologies that can improve patient outcomes. BSMH is committed to bringing quality care to more people by eliminating time-consuming data roadblocks for their staff.” For further information, please contact: Silvie CasanovaPhilips North America Tel: +1 781 879-0692 Email: silvie.casanova@philips.com Mark GrovesPhilips External RelationsTel: +31 631 639 916Email: mark.groves@philips.com Emma SwannBon Secours Mercy HealthTel: +1 804 837-0413Email: Emma_swann@bshsi.org About Bon Secours Mercy HealthBon Secours Mercy Health (BSMH) is one of the 20 largest health systems in the United States and the fifth-largest Catholic health system in the country. The ministry’s quality, compassionate care is provided by more than 60,000 associates serving communities in Florida, Kentucky, Maryland, New York, Ohio, South Carolina, and Virginia, as well as throughout Ireland. Bon Secours Mercy Health provides care for patients more than 11 million times annually through its network of more than 1,200 care sites, which includes 49 hospitals. In 2023, BSMH provided more than $600 million dollars in community investments across five states, ensuring that cost is not a barrier to health care for our patients in need. In addition to charity care, BSMH invests in programs that address chronic illness, affordable housing, access to healthy food, education and wellness programs, transportation, workforce development and other social determinants of health that directly affect the communities we serve. The Mission of Bon Secours Mercy Health is to extend the compassionate ministry of Jesus by improving the health and well-being of its communities and bring good help to those in need, especially people who are poor, dying and underserved. For more information, visit https://bsmhealth.org/. About Royal PhilipsRoyal Philips (NYSE: PHG, AEX: PHIA) is a leading health technology company focused on improving people’s health and well-being through meaningful innovation. Philips’ patient- and people-centric innovation leverages advanced technology and deep clinical and consumer insights to deliver personal health solutions for consumers and professional health solutions for healthcare providers and their patients in the hospital and the home. Headquartered in the Netherlands, the company is a leader in diagnostic imaging, ultrasound, image-guided therapy, monitoring and enterprise informatics, as well as in personal health. Philips generated 2023 sales of EUR 18.2 billion and employs approximately 69,100 employees with sales and services in more than 100 countries. News about Philips can be found at www.philips.com/newscenter.
Attachment
Doctor monitoring patient health
Philips Zenition 90 Motorized receives FDA 510(k) clearance, helping clinicians deliver high quality care with a high-powered and fast motorized mobile C-arm
June 17, 2024 Intuitive motorization for greater control and high-power (25 kW [1]) for state-of-the-art image quality supports complex vascular needs and a full range of clinical proceduresAutomated workflows contribute to greater flexibility and independence for clinicians, providing more time to focus on achieving the best possible outcomes for patients Amsterdam, the Netherlands – Royal Philips (NYSE: PHG, AEX: PHIA), a global leader in health technology, today announced the launch of Philips Image Guided Therapy Mobile C-arm System 9000 – Zenition 90 Motorized, designed to help clinicians deliver high-quality care to more patients. Philips is partnering with its customers to improve productivity. The new mobile C-arm with expanded capabilities is designed to meet complex vascular needs, but also a range of clinical procedures such as cardiac interventions, pain management and urology. Philips will be showcasing its newly introduced mobile C-arm at the 2024 Society for Vascular Surgery Annual Meeting, June 19-22, in Chicago. Increased control and efficiency with automated workflowsThe Philips Zenition Image-Guided Therapy Mobile C-arm Systems bring together innovations in image capture and processing, ease-of-use, and versatility, many of which were pioneered on Philips’ highly successful image guided therapy platform Azurion. Motorized and impressively fast, the Zenition 90 Motorized is an intuitive C-arm that allowsclinicians to control it from the table-side with user-friendly controls and time-saving features – empowering the clinician with greater flexibility and independence. It delivers state-of-the-art image quality for the most challenging procedures and is designed to meet complex procedural needs. The system allows greater clinical efficiency thanks to its automated workflows, the image controls via the Touch Screen Module and the advanced software solutions. “During complex procedures, it’s vital to be able to rely on surgical imaging systems. As clinicians navigate their way through challenging anatomy, the priority is to quickly visualize small anatomical details while limiting X-ray dose,” said Mark Stoffels, Business Leader Philips Image Guided Therapy Systems. “The new Zenition 90 Motorized empowers medical teams to confidently perform a wide range of interventions while achieving the best possible outcome for their patients.” In independent hands-on usability studies of clinicians from the US and EU with the Zenition 90 Motorized in simulated environments; 100% of users said that with the Table Side Operator, they have complete control over C-arm movements [2] and 97% report that workflow features such as Automatic Vascular Outlining will help save time during procedures [3]. As part of its commitment to sustainability and providing customers with responsible choices, Philips leveraged its EcoDesign process for the Zenition 90 Motorized to improve product life by 25% and power efficiency by 13% [4]. Philips latest image guided therapy mobile C-arm system is also available in a non-motorized configuration.[1]Also available in 15 kW[2] Results obtained during claims substantiation study performed in June 2022 and May 2023 by Use-Lab GmbH, an independent company. Response is based on 25 physicians from the EU and the US, who answered a questionnaire after a usability study with additional hands-on time with the system.[3] Results obtained during claims substantiation study performed in June 2022 and May 2023 by Use-Lab GmbH, an independent company. Response is based on 49 clinicians from the EU and the US, who answered a questionnaire after a usability study with additional hands-on time with the system.[4] Compared to its predecessor, Zenition 70- Zenition 90 Motorized and Zenition 90 are available for sales in a limited number of countries.- Some features are optional for Zenition 90 Motorized and Zenition 90.- Actual product representation may vary. For further information, please contact:Joost MalthaPhilips External RelationsTel. : +31 6 10558116E-mail: joost.maltha@philips.com About Royal PhilipsRoyal Philips (NYSE: PHG, AEX: PHIA) is a leading health technology company focused on improving people’s health and well-being through meaningful innovation. Philips’ patient- and people-centric innovation leverages advanced technology and deep clinical and consumer insights to deliver personal health solutions for consumers and professional health solutions for healthcare providers and their patients in the hospital and the home. Headquartered in the Netherlands, the company is a leader in diagnostic imaging, ultrasound, image-guided therapy, monitoring and enterprise informatics, as well as in personal health. Philips generated 2023 sales of EUR 18.2 billion and employs approximately 69,100 employees with sales and services in more than 100 countries. News about Philips can be found at www.philips.com/newscenter.
Attachments
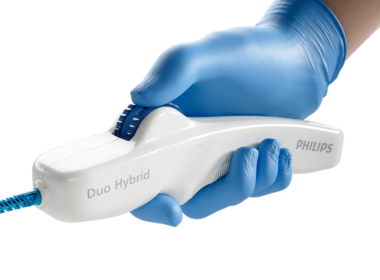
Philips launches Duo Venous Stent System for treatment of symptomatic venous outflow obstruction
June 12, 2024 Sanger Heart & Vascular Institute, Atrium Health, treated the first patient with the new implantable medical device following FDA premarket approval Amsterdam, the Netherlands – Royal Philips (NYSE: PHG, AEX: PHIA), a global leader in health technology, today announced the first implant of the Duo Venous Stent System, an implantable medical device indicated to treat symptomatic venous outflow obstruction in patients with chronic venous insufficiency (CVI), following premarket approval (PMA) from the U.S. Food and Drug Association (FDA). On June 11, Dr Erin Murphy – vascular surgeon and director of the Venous and Lymphatic Program at the renowned Sanger Heart & Vascular Institute, Atrium Health, in Charlotte, N.C., and an investigator in the VIVID study, which contributed to the device’s FDA approval – successfully used the Duo Venous Stent System for the first time outside of a clinical trial. Impacting 25 million people globally [1], deep venous disease results from venous thromboembolism, a condition that occurs when a blood clot forms in the vein [2]. It is the third most common cardiovascular disease [2]. Deep venous anatomy and obstructions can present a multitude of complexities and mechanical challenges. Engineered for the unique demands of venous anatomy and obstructions, the Duo Venous Stent System is comprised of two stents – Duo Hybrid and Duo Extend – of various sizes. Duo Hybrid has a distinct integrated design that combines multiple zones of differing mechanical properties into a single stent [3]. For long lesions, Duo Extend smoothly overlaps with the Duo Hybrid to extend therapy. These two stents are designed to work together and minimize the risk of stent fracture and corrosion, while providing an option to stent within caudal veins with smaller diameters [3]. “Duo is the first stent that offers a differential design for the challenges of venous anatomy – a focal area that withstands the forces of compression as well as the flexibility to accommodate curvature of the vessel,” said Dr Kush Desai, a highly regarded Interventional radiologist and associate professor of Radiology, Surgery and Medicine at Northwestern University in Chicago, as well as a leading enroller and investigator for the VIVID study. “Consequently, Philips is well positioned to support CVI treatment by offering a robust portfolio of medical technology that includes both intravascular ultrasound and a differentiated venous stenting system.” VIVID studyThe VIVID study is a global, prospective, multi-center, single-arm, non-blinded clinical trial conducted in the U.S. and Poland, evaluating the safety and efficacy of the Philips Duo Venous Stent System in the treatment of nonmalignant iliofemoral occlusive disease. It enrolled 162 subjects at 30 centers with three patient populations – non-thrombotic iliac vein lesion (NIVL), post-thrombotic syndrome (PTS) and acute deep vein thrombosis (aDVT). The VIVID study is now in 36-month follow-up and upon FDA PMA approval transitioned from an investigational device exemption (IDE) study to a post-approval study (PAS): NCT04580160. The VIVID study met all of its primary safety and efficacy performance goals. The 12-month effectiveness endpoint for primary patency reached 90.2%, which exceeded the performance target goal of 77.3%. The 12-month primary safety endpoint of 98.7% also exceeded the corresponding performance goal of 89%. In addition, quality of life and venous functional assessments that were performed in the VIVID study – including Clinical-Etiology-Anatomy-Pathophysiology (CEAP), Venous Clinical Severity Score (VCSS), Villalta, EQ-5D-3L and VEINES scores – showed sustained improvements compared to baseline at 12 months. “The VIVID study’s 12-month results demonstrate the safety and efficacy of the Duo Venous Stent System in the treatment of chronic venous insufficiency, a vascular condition affecting millions of people worldwide,” said principal investigator Dr Mahmood Razavi, M.D., an interventional radiologist with St. Joseph Vascular in Orange County, Calif. “Duo represents a meaningful addition to the tools that clinicians can use to treat CVI patients,” Dr Razavi added, “especially when used in conjunction with intravascular ultrasound, or IVUS. Ultimately, the new device promises to enable excellent clinical outcomes and drive significant quality of life improvements.” The VIVID study was the first clinical trial to mandate IVUS use to aid in lesion assessment and stent sizing prior to device implantation. According to prior published research, IVUS supports accurate diagnosis of venous disease and has been shown to change 57% of treatment plans compared to venography alone [4]. Led by Philips, intravascular imaging is used in more than 70% of venous stent procedures [5]. “The launch of the Duo Venous Stent System represents another step forward in achieving our aspiration to innovate interventional procedures with advanced medical technology,” said Heather Hudnut Page, Vice President and Business Leader of Peripheral Vascular at Philips. “In this context, we look forward to bringing the combined offering of intravascular ultrasound and Duo to the interdisciplinary teams – from vascular surgeons to interventional radiologists and interventional cardiologists – who share our overarching goal of enhancing patient care.” [1] Market Model Sources- DVD: 1 US Physician Quant Survey- Leveraged for NIVL prevalence assumption as ~25% of Symptomatic DVD 2 Thrombosis: a major contributor to the global disease burden. J Thromb Haemost 2014; 12: 1580–90. – Leveraged for DVT incidence in some countries (ex. EU5) 3 DRG VTE Epidemiology Reports- Leveraged for DVT incidence in some countries 4 Inari Medical presentation and Khan, SR, Arch Intern Med 2004- Leveraged for assumption of PTS.[2] Scheres LJJ, Lijfering WM, Cannegieter SC. Current and future burden of venous thrombosis: Not simply predictable. Res Pract Thromb Haemost. 2018 Apr 17;2(2):199-208. doi: 10.1002/rth2.12101. PMID: 30046722; PMCID: PMC6055567.[3] Data on file: D062749[4] Gagne PJ, Tahara RW, Fastabend CP, et al. Venography versus intravascular ultrasound for diagnosing and treating iliofemoral vein obstruction. J Vasc Surg Venous Lymphat Disord. 2017;5:678-687.[5] Divakaran S, Meissner MH, Kohi MP, et al. Utilization of and Outcomes Associated with Intravascular Ultrasound during Deep Venous Stent Placement among Medicare Beneficiaries. J Vasc Interv Radiol. 2022;33(12):1476-1484.e2. doi:10.1016/j.jvir.2022.08.018 Regulatory disclosures Developed by Vesper Medical, Inc., a wholly owned subsidiary of Philips, the Duo Venous Stent System is being marketed under the Philips brand. Its FDA approval order, along with related regulatory information, can be found here: PMA approval for Duo Venous Stent System. Caution: Federal law restricts this device to sale by or on the order of a licensed healthcare practitioner. For further information, please contact:Joost MalthaPhilips Global Press OfficeTel. : +31 (6) 1055 8116Email : joost.maltha@philips.com About Royal PhilipsRoyal Philips (NYSE: PHG, AEX: PHIA) is a leading health technology company focused on improving people’s health and well-being through meaningful innovation. Philips’ patient- and people-centric innovation leverages advanced technology and deep clinical and consumer insights to deliver personal health solutions for consumers and professional health solutions for healthcare providers and their patients in the hospital and the home. Headquartered in the Netherlands, the company is a leader in diagnostic imaging, ultrasound, image-guided therapy, monitoring and enterprise informatics, as well as in personal health. Philips generated 2023 sales of EUR 18.2 billion and employs approximately 69,100 employees with sales and services in more than 100 countries. News about Philips can be found at www.philips.com/newscenter.
Attachments
Duo Venous Stent System
Duo Venous Stent System 2
Philips expands global access to its ground-breaking 3D intracardiac echocardiography to Hong Kong
Philips expands global access to its ground-breaking 3D intracardiac echocardiography to Hong Kong Hong Kong selected as the pioneering international location for integrating Philips’ VeriSight Pro 3D Intracardiac Echocardiography (ICE) catheter into routine clinical practice International roll-out underscores Philips’ commitment to driving healthcare innovation and enhancing patient outcomes worldwide Amsterdam, […]
Philips ePatch and AI analytics platform rolled out to 14 hospitals across Spain to monitor heart patients
May 22, 2024 Philips wearable ePatch and AI analytics platform help detect potentially life-threatening irregular heartbeatsThe solution helps University Hospital Vall d’Hebron achieve reduced average length of stay, relieve emergency room pressure, and reduce costs Amsterdam, the Netherlands, and Madrid, Spain – Royal Philips (NYSE: PHG, AEX: PHIA), a global leader in health technology, today announced the successful nationwide rollout of its ambulatory cardiac monitoring service in Spain using its unique wearable ePatch paired with its AI-driven Cardiologs analytics platform. 14 Spanish healthcare providers across the country are now using the company’s ePatch extended wear Holter monitors to detect life-threatening heart arrhythmias such as atrial fibrillation (AF). The solution has been proven to detect heart arrhythmias missed by traditional Holter monitors, enhance patient comfort, improve care access, and potentially better clinical outcomes, in addition to reducing overall costs. Philips’ ePatch provides reliable data for up to 14 days of continuous monitoring. When paired with Philips’ AI-driven Cardiologs analytics platform, the solution empowers the hospitals’ cardiology and neurology teams with an end-to-end solution that enhances their ability to detect and diagnose AF – a potentially life-threatening [1] cardiac arrhythmia and also the world’s most common. AF significantly increases the risk of stroke, dementia, and heart failure, yet often goes undetected due to its lack of noticeable symptoms and infrequent occurrence intervals. “The key advantage for healthcare professionals lies in its user-friendly interface and high-quality 14-day continuous recording capabilities,” said Dr. Jorge Pagola, Neurologist Postdoctoral Researcher at University Hospital Vall d’Hebron, Barcelona, Spain. “Applied as a chest patch in just a few minutes, it seamlessly integrates an analysis program, facilitating swift examination of recordings. Thanks to its AI-based analysis assistant, AF events can be classified for rapid review by our team. Patients experience enhanced comfort as they are free from cumbersome cables of the conventional Holter, allowing them to dress, shower, and carry out their daily activities without any hindrance.” Dr. Jorge Pagola further explains, “Using the ePatch program, we expedited hospital discharge for 80 patients in 2023. This initiative led to a reduced average length of stay, relieved emergency room pressure, and an estimated total cost reduction of € 28.800 in 2023 [2].” Pilot programs in major hospitals across SpainIn addition to Hospital Vall d’Hebron, where Philips’ ePatch is being used to detect post-discharge AF in patients, pilot projects demonstrating the device’s effectiveness in cardiology and neurology are being conducted at other major hospitals in Madrid, Barcelona, Bilbao, Alicante, Madrid, Cadiz, and Navarra [3]. Applications include monitoring patients for AF after cardiac ablation therapy or heart valve replacement procedures, and studies of the link between magnesium insufficiency and AF. In some Spanish hospitals, the ease-of-use and cost benefits of Philips ePatch are helping reduce waiting lists that built up during the COVID-19 pandemic, making a tangible difference to people’s access to care. In total, more than 1500 patients are currently being monitored with Philips ePatch devices in Spain. “This innovative new service allows clinical teams to conveniently monitor patients as they go about their everyday activities for extended periods of time, collecting the real-life data that helps reveal the patient’s true condition,” said Miquel Barras, Ambulatory Monitoring & Diagnostics Lead for Philips in Spain. In a recent US study comparing the diagnostic abilities of 7-day and 14-day monitoring with Philips’ extended wear ePatch compared to conventional 24-hour Holter monitoring, the ePatch detected 2x more clinically significant heart arrhythmias during 7 days of monitoring and more than 2.5x as many over 14 days [4]. [1] Heart.org[2] Data on file in hospital Vall d’Hebron[3] Madrid: Hospital Universitario Fundación Jiménez Díaz, Hospital Universitario Infanta Elena, Hospital de La Princesa, University Hospital Quironsalud Madrid, Hospital Universitario Rey Juan Carlos. Barcelona: Vall d’Hebron University Hospital, Hospital Clínic de Barcelona, Hospital de Sant Joan Despí Moisès Broggi, Hospital Sant Joan de Déu Barcelona, Hospital Universitari de Bellvitge, Hospital de Sant Pau. Bilbao: Hospital Universitario Cruces. Alicante: Hospital General Universitario de Elche. Cadiz: Hospital Universitario De Jerez. Navarra: Hospital General de Navarra.[4] Parikh, P, Grigoriadis, C, Dunn, A. et al. Diagnostic Yield of 24-hour Holter vs 7-day and 14-day ePatch Extended Wear Holter. J Am Coll Cardiol. 2023 Mar, 81 (8_Supplement) 149. Research conducted by Philips ECG Solutions.https://doi.org/10.1016/S0735-1097(23)00593-4 For further information, please contact: César García RequenaPhilips IbéricaTel: +34 670 264 471Email: cesar.garcia.requena@philips.com Joost Maltha External RelationsTel: +31 6 10 55 8116 Email: joost.maltha@philips.com About Royal Philips Royal Philips (NYSE: PHG, AEX: PHIA) is a leading health technology company focused on improving people’s health and well-being through meaningful innovation. Philips’ patient- and people-centric innovation leverages advanced technology and deep clinical and consumer insights to deliver personal health solutions for consumers and professional health solutions for healthcare providers and their patients in the hospital and the home. Headquartered in the Netherlands, the company is a leader in diagnostic imaging, ultrasound, image-guided therapy, monitoring and enterprise informatics, as well as in personal health. Philips generated 2023 sales of EUR 18.2 billion and employs approximately 69,100 employees with sales and services in more than 100 countries. News about Philips can be found at www.philips.com/newscenter.
Attachments
Philips presents study results at Heart Rhythm Annual Meeting demonstrating benefits of its AI-powered cardiac monitoring solutions
May 17, 2024
Bringing confident diagnosis to more patients at low cost: Philips launches new AI-enabled CT 5300 at #ECR2024
February 29, 2024 New CT 5300 system provides more accurate and reliable imaging results to better manage increase in complex cardiac cases where precise patient diagnosis is crucial Philips’ latest innovation leverages the power of AI at every stage in CT scanner operation to enhance productivity and diagnostic capabilities of […]
Philips launches high-power and fast motorized mobile C-arm, the Zenition 90 Motorized, designed to help surgeons deliver high-quality care to patients at #ECR2024
Amsterdam, the Netherlands and Vienna, Austria – Royal Philips (NYSE: PHG, AEX: PHIA), a global leader in health technology, today announced the launch of Philips Image Guided Therapy Mobile C-arm System 9000 – Zenition 90 Motorized, designed to help surgeons deliver high-quality care to more patients. The new mobile C-arm with expanded capabilities […]