HANGZHOU, China, Aug. 30, 2023 /PRNewswire/ — Zylox-Tonbridge Medical Technology Co., Ltd. (2190.HK, hereinafter referred to as “Zylox-Tonbridge” or the “Company”) announced that its ZENFlow Tiger™ LD PTA Dilatation Catheter (hereinafter referred to as “ZENFlow Tiger™”), a product independently developed by the Company, has received marketing approval from ANVISA. The Company expects […]
Tag: PTA
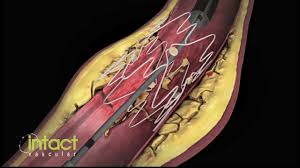
Positive Twenty-Four Month Tack Optimized Balloon Angioplasty (“TOBA”) Single Center Results Presented at VEITHsymposium™ Conference
WAYNE, Pa.–(BUSINESS WIRE)–Intact Vascular, Inc., a developer of medical devices for minimally-invasive peripheral vascular procedures, today announced that positive single center twenty-four month results from its Tack Optimized Balloon Angioplasty (TOBA) clinical study were presented at the VEITHsymposium™ 2017 conference by Dr. Christian Wissgott, Assistant Director, at Westküstenklinikum Heide in Heide, Germany. The TOBA study enrolled […]
SurModics (SRDX) Nabs Global Approvals of .014″ Low-Profile PTA Balloon Dilation Catheter
EDEN PRAIRIE, Minn.–(BUSINESS WIRE)–Surmodics, Inc. (NASDAQ: SRDX), a leading provider of medical device and in vitro diagnostic technologies to the healthcare industry, announced it has received U.S. Food and Drug Administration (FDA) 510(k) and CE Mark clearance for its .014” low-profile percutaneous transluminal angioplasty (PTA) balloon dilation catheter, designed for […]
AV Medical’s Chameleon receives expanded indication to include infusion of diagnostic or therapeutic fluids
TEL AVIV, Israel–(BUSINESS WIRE) AV Medical Technologies announced on 27 February 2017 that its Chameleon PTA balloon catheter has received FDA clearance for an expanded indication to include infusion of diagnostic or therapeutic fluids. “The Chameleon balloon offers advantages for experienced operators as well as those training in dialysis access […]