First Patient Enrollment in AGILITY Study Begins Investigations of Safety and Effectiveness of BD Vascular Covered Stent for Treatment of PAD FRANKLIN LAKES, N.J., March 4, 2024 /PRNewswire/ — BD (Becton, Dickinson and Company) (NYSE: BDX), a leading global medical technology company,…
Tag: PVD
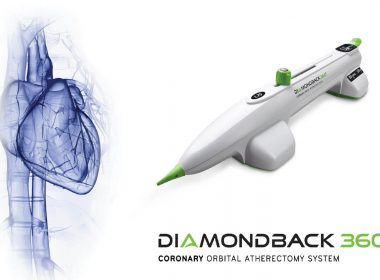
Cardiovascular Systems, Inc. Announces First Patients Treated from Radial Access Using Its Diamondback 360 Extended Length Peripheral Orbital Atherectomy Device
Feb. 14, 2018 12:00 UTC Device provides alternative access point to treat patients suffering from peripheral artery disease ST. PAUL, Minn.–(BUSINESS WIRE)– Cardiovascular Systems, Inc. (CSI®) (NASDAQ: CSII), a medical device company developing and commercializing innovative interventional treatment systems for peripheral and coronary artery disease, announced today that the first patients were […]
Global Peripheral Vascular Devices Market Analysis 2017 and Forecasts 2018-2022 by Type – A Potential $12.6 Billion Market – Research and Markets
DUBLIN–(BUSINESS WIRE)–The “Peripheral Vascular Devices Market by Type (Angioplasty Balloon, Stent, Catheters, Plaque Modification, Hemodynamic Flow Alteration, IVC Filters, Guidewires) – Global Forecast to 2022” report has been added to Research and Markets’ offering. The global peripheral vascular devices market is expected to reach USD 12.63 Billion by 2022 from USD 9.09 Billion in […]
Intact Vascular Announces Enrollment Of First European Patient In Tack Optimized Balloon Angioplasty II Below The Knee (TOBA II BTK) Clinical Trial With The Tack Endovascular System
WAYNE, Pa.–(BUSINESS WIRE)–Intact Vascular, Inc., a developer of medical devices for minimally invasive peripheral vascular procedures, today announced that its Tack Optimized Balloon Angioplasty II Below the Knee (TOBA II BTK) clinical trial has commenced enrollment in Europe, with the first patient treated by Professor Dr. Marianne Brodmann and Dr. Peter Reif at Medical University Graz, Austria. […]
Avera Names Myles Greenberg MD CEO Of Alucent Medical
SIOUX FALLS, S.D.–(BUSINESS WIRE)–Myles D. Greenberg, MD, was named President and CEO of Alucent Medical, Inc., a new company licensed to develop a novel drug/device combination therapy for the treatment of peripheral vascular disease (PVD). The treatment, Natural Vascular Scaffolding (NVS)TM, has recently gained FDA approval to move forward with […]