Proceeds to Fund the Company’s Worldwide Commercial Expansion Series D fundraising led by MicroPort YOKNEAM, Israel–(BUSINESS WIRE)–Rapid Medical, a company focused on the development of responsive, adjustable neurovascular devices, announced today that it has completed an oversubscribed Series D financing of $50M. The proceeds will be used to support the […]
Tag: Rapid Medical
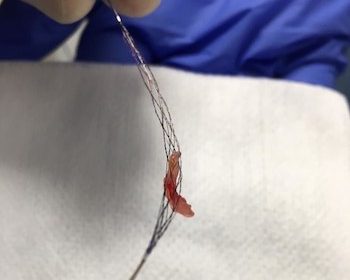
Rapid Medical Receives FDA Clearance for the First Adjustable Stent Retriever for Use in Ischemic Stroke Treatment
TIGERTRIEVER™ uses Rapid Medical’s proprietary technology to significantly improve the effectiveness of minimally invasive clot removal in the brain YOKNEAM, Israel & MIAMI–(BUSINESS WIRE)–Rapid Medical, developer of responsive, adjustable neurovascular devices, announces the FDA clearance of its TIGERTRIEVER™ revascularization device for use in the treatment of ischemic stroke. TIGERTRIEVER is the first […]
TIGER Trial Demonstrates High Safety and Efficacy for World’s First Adjustable Thrombectomy Device
Rapid Medical’s TIGERTRIEVER™ advances stent retriever technology for stroke treatment YOKNEAM, Israel & MIAMI–(BUSINESS WIRE)–The first adjustable, fully visible stent retriever shows superiority over conventional stent retrievers in a multicenter trial. The late breaking results were recently presented at the International Stroke Conference and published online in the journal Stroke. The TIGER Trial demonstrates a […]
Rapid Medical Announces Appointment of James Romero as President, Americas
Experienced Neurovascular Device Executive will Lead Commercialization in US, Canada and LATAM YOKNEAM, Israel & FORT LAUDERDALE, Fla.–(BUSINESS WIRE)–Rapid Medical, a company focused on the development of the first class of responsive, adjustable neurovascular devices, announces James Romero as the company’s President, Americas. Mr. Romero will oversee the growth and […]
Rapid Medical Receives FDA Clearance for the World’s First Steerable Neurovascular Guidewire
DRIVEWIRE™ provides efficient access in difficult neuro and peripheral vascular procedures YOKNEAM, Israel & MIAMI–(BUSINESS WIRE)–Rapid Medical, a company focused on the development of the first class of responsive, adjustable neurovascular devices, announces the FDA clearance of DRIVEWIRE, a novel guidewire with a steerable distal tip that handles complex anatomical […]
Rapid Medical Expands Comaneci™ CE Mark Indication to Include Vasospasm Treatment
Comaneci is the first adjustable mesh device indicated for the endovascular treatment of vasospasm YOKNEAM, Israel, Nov. 23, 2020 /PRNewswire/ — Rapid Medical, a company focused on the development of next-generation neurovascular devices, has announced CE Mark approval for the expanded indication of Comaneci to treat cerebral vasospasm. Comaneci is the first adjustable remodeling […]
Rapid Medical Receives CE Mark for TIGERTRIEVER™ XL
TIGERTRIEVER XL is the largest stentriever available, designed to safely remove clots from intracranial vessels up to 9mm in diameter YOKNEAM, Israel, July 27, 2020 /PRNewswire/ — Rapid Medical, a company focused on the development of next generation neurovascular devices, has announced that it received CE Mark for TIGERTRIEVER XL. In addition, the first […]
Rapid Medical Announces Ahead of Schedule Completion of Patient Enrollment for the TIGER Clinical Study for TIGERTRIEVER Thrombectomy Device
YOKNEAM, Israel, April 7, 2020 /PRNewswire/ — Rapid Medical, a company focused on the development of next generation neurovascular interventional devices, has announced that it has completed enrollment in the TIGER (Treatment with Intent to Generate Reperfusion) study ahead of the planned scheduled. This is a US based, multi-center study of the performance of TIGERTRIEVER, the company’s […]
Rapid Medical Announces FDA Approval of Novel Temporary Aneurysm Embolization Assist Device
YOKNEAM, Israel, May 7, 2019 /PRNewswire/ — Rapid Medical, a company focused on the development of next generation neurovascular devices, today announced that its Comaneci device received FDA clearance as a Temporary Coil Embolization Assist Device. The Comaneci is the first and only device in a new category of temporary coil embolization assist devices. […]
Rapid Medical Raises $20 Million in New Funding to Support Clinical and Commercialization of First-in-class Stroke Treatment Products
YOKNEAM, Israel, April 23, 2019 /PRNewswire/ — Rapid Medical, a company focused on the development of interventional neurovascular devices, announced today that it has completed an oversubscribed Series C financing of $20 million. The proceeds will be used for the completion of the TIGER U.S. IDE study and to support accelerating commercial growth […]