Bridge Occlusion Balloon Catheter Model 590-001 by Spectranetics: Class I Recall – Risk of Blocked Guidewire Lumen Preventing Balloon Utilization ISSUE: Spectranetics is recalling its Bridge Occlusion Balloon Catheter due to the possibility of a blocked guidewire lumen in some device units. If a device with a blocked guidewire lumen […]
Tag: recall
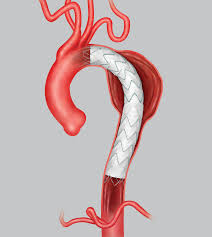
Cook has Class I Recall for Zenith Alpha Thoracic Graft
Cook said to be recalling this device because of blood clots that can form within the graft after implantation. Zenith Alpha Thoracic Endovascular Graft by Cook Medical: Class I Recall – Potential Formation of Thrombus Inside Device AUDIENCE: Risk Manager, Cardiology, Surgery, Patient ISSUE: Cook Medical Inc. is recalling the […]
Penumbra initiates recall of 3D Revascularation Device
Penumbra Inc. Recalls 3D Revascularization Device Due to Wire Material That May Break or Separate During Use Penumbra Inc. is recalling the Penumbra 3D Revascularization device because there is a risk of the delivery wire breaking or separating during use. Fractured pieces of the delivery wire could be left inside […]
Datascope Corp./MAQUET Issues Worldwide Voluntary Recall of the System CS100, CS100i and CS300 Intra-Aortic Balloon Pumps for Potential Electrical Test Failure Code
WAYNE, N.J., June 16, 2017 /PRNewswire/ — Datascope Corp. is voluntarily performing a worldwide field correction of certain Intra-Aortic Balloon Pumps (IABPs) sold by Datascope Corp. for a potential electrical test failure code. AFFECTED PRODUCT PART NUMBER CS100i IABP CS100 IABP CS300 IABP 0998-UC-0446HXX; 0998-UC-0479HXX 0998-00-3013-XX; 0998-UC-3013-XX 0998-00-3023-XX; 0998-UC-3023-XX This […]