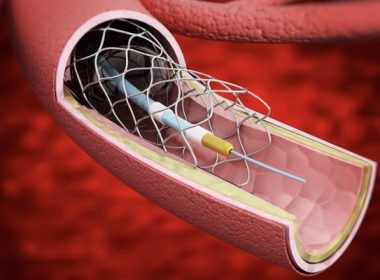
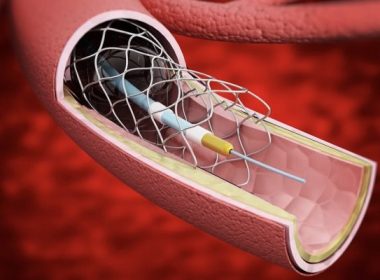
SAN DIEGO, Sept. 27, 2022 /PRNewswire/ — REVA Medical, LLC, a leader in bioresorbable polymer technologies for vascular applications, today announced that enrollment in the MOTIV pivotal trial has been initiated at clinical centers in both the U.S. and Europe. The study will evaluate the use of the MOTIV® Sirolimus-Eluting Bioresorbable Vascular Scaffold for […]
Tag: REVA Medical
REVA MEDICAL ANNOUNCES CLOSING OF STRATEGIC FINANCING
SAN DIEGO, Aug. 9, 2022 /PRNewswire/ — REVA Medical, LLC. (“REVA” or the “Company”), a leader in bioresorbable polymer technologies for vascular applications, is pleased to announce the closing of a $45 million Series B equity financing. This funding was led by a global strategic investor with deep experience in medical devices, as well […]
REVA Receives Interim Funding Commitment and Announces Leadership Changes
SAN DIEGO, March 24, 2019 (GLOBE NEWSWIRE) — REVA Medical, Inc. (ASX: RVA) (“REVA” or the “Company”) said today that it has secured a letter of commitment for up to $3 million of debt financing to fund operations on an interim basis. The funding will be made available by existing […]
REVA Announces Extension to Voluntary Suspension of Trading
SAN DIEGO, Feb. 24, 2019 (GLOBE NEWSWIRE) — REVA Medical, Inc. (ASX: RVA) (“REVA” or the “Company”) said today that on 25 February 2019 (AEDT), the Australian Securities Exchange issued a market announcement indicating that the securities of the Company will remain suspended from quotation at the Company’s request until […]
REVA Expands Geographic Footprint to Seven New Countries
SAN DIEGO, Jan. 07, 2019 (GLOBE NEWSWIRE) — REVA Medical, Inc. (ASX: RVA) (“REVA” or the “Company”), a leader in bioresorbable polymer technologies for vascular applications, announced the geographic expansion of its commercial operations in seven European countries with the addition of four new distribution partnerships. These partnerships will allow […]
Leigh Elkolli Promoted to Chief Financial Officer at REVA Medical
SAN DIEGO, Jan. 03, 2019 (GLOBE NEWSWIRE) — REVA Medical, Inc. (ASX: RVA) (“REVA” or the “Company”), a leader in bioresorbable polymer technologies for vascular applications, announced the promotion of Leigh Elkolli to the position of Chief Financial Officer and Corporate Secretary, effective January 4, 2019. She will replace Brandi […]
New Data for Fantom in Heart Attack Patients to be Presented at the TCT 2018 Conference
SAN DIEGO, Aug. 15, 2018 (GLOBE NEWSWIRE) — REVA Medical, Inc. (ASX: RVA) (“REVA” or the “Company”), a leader in bioresorbable polymer technologies for vascular applications, will present new data on the use of the Fantom bioresorbable scaffold in patients experiencing heart attacks during the upcoming Transcatheter Cardiovascular Therapeutics (“TCT”) […]
Reva Expands Commercial Access to Fantom With New Distribution Partnership in Italy
SAN DIEGO, July 15, 2018 (GLOBE NEWSWIRE) — REVA Medical, Inc. (ASX:RVA) (“REVA” or the “Company”), a leader in bioresorbable polymer technologies for vascular applications, is expanding commercial access to the Fantom® Bioresorbable Scaffold with a new distribution partnership in Italy. REVA will work with Bio Vascular Group S.R.L. (“Bio Vascular”) […]
REVA to Hold Annual General Meeting of Stockholders
SAN DIEGO, May 09, 2018 (GLOBE NEWSWIRE) — REVA Medical, Inc. (ASX:RVA) (“REVA” or the “Company”) will hold its 2018 Annual General Meeting of Stockholders (the “AGM”) on Wednesday, May 16, 2018 at 4:00 p.m. US PDT (which is Thursday, 17 May 2018 at 9:00 a.m. AEST). The meeting will […]
REVA ANNOUNCES CE MARK AND FIRST IMPLANT OF THE FANTOM ENCORE BIORESORBABLE SCAFFOLD
2.5 millimeter Diameter Size with Market-Leading 95 micron Strut Profile Secures Early Approval San Diego, California (Monday, February 26, 2018 – PST) – REVA Medical, Inc. (ASX: RVA) (“REVA” or the “Company”), a leader in bioresorbable polymer technologies for vascular applications, announces CE Mark of the 2.5 millimeter diameter size […]