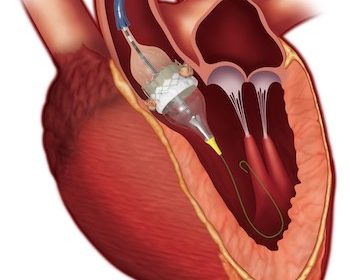
November 25, 2024 03:30 AM Eastern Standard Time LONDON–(BUSINESS WIRE)–Edwards Lifesciences (NYSE: EW) today announced one-year data highlighting the continued outstanding performance of its SAPIEN 3 Ultra RESILIA valve. The data were presented at PCR London Valves 2024 and simultaneously published in the Journal of the American College of Cardiology (JACC): […]
Tag: Sapien 3
Edwards Launches SAPIEN 3 With Alterra Prestent in Europe for Transcatheter Pulmonic Valve Implantation
September 16, 2024 07:01 PM Eastern Daylight Time NYON, Switzerland–(BUSINESS WIRE)–Edwards Lifesciences today announced the launch in Europe of the SAPIEN 3 transcatheter pulmonary valve implantation (TPVI) system with Alterra adaptive prestent, expanding minimally invasive treatment options to a broader range of patients with congenital heart conditions. “I am proud […]
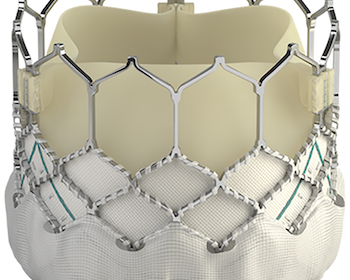
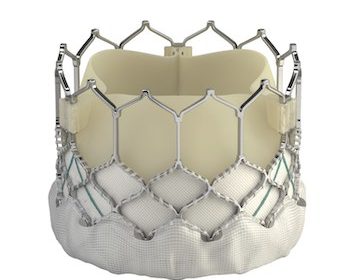
Edwards Launches the SAPIEN 3 Ultra RESILIA Valve in Europe With Technology to Enhance Durability
NYON, Switzerland–(BUSINESS WIRE)–Edwards Lifesciences today announced the European launch of the SAPIEN 3 Ultra RESILIA valve, the only transcatheter aortic heart valve to incorporate the company’s breakthrough RESILIA tissue technology, designed to extend the valve’s durability.* Edwards’ SAPIEN 3 Ultra RESILIA valve recently received CE Mark† for use in patients with […]
Edwards Receives FDA Approval for SAPIEN 3 with Alterra Prestent for Transcatheteter Pulmonic Valve Replacement
IRVINE, Calif., Dec. 20, 2021 /PRNewswire/ — Edwards Lifesciences (NYSE: EW) today announced it received approval from the U.S. Food and Drug Administration (FDA) for the use of the Edwards SAPIEN 3 transcatheter valve with the Alterra adaptive prestent (SAPIEN 3 with Alterra) for patients with severe pulmonary regurgitation. The Edwards SAPIEN 3 […]
TAVR with SAPIEN 3 Demonstrated as Economically Dominant Treatment Strategy Compared to Surgery in Partner 3 Analysis
IRVINE, Calif., Nov. 5, 2021 /PRNewswire/ — Edwards Lifesciences (NYSE: EW) today announced that a cost-effectiveness analysis comparing transcatheter aortic valve replacement (TAVR) to surgery demonstrated that TAVR with SAPIEN 3 is an economically dominant treatment strategy, offering improved outcomes and reduced cost. This analysis from the PARTNER 3 trial was presented during the […]
Real-World Data Confirm Excellent Results For Bicuspid Patients Treated With Edwards SAPIEN 3 TAVR
At 1 year, outcomes similar to overall cohort of patients IRVINE, Calif., May 18, 2021 /PRNewswire/ — Edwards Lifesciences (NYSE: EW) today announced the results of a real-world study comparing outcomes for patients with bicuspid aortic stenosis (AS) who were treated with SAPIEN 3 and SAPIEN 3 Ultra Transcatheter Aortic Valve Replacement (TAVR) and […]
Edwards SAPIEN 3 Transcatheter Heart Valve Receives Approval In China
IRVINE, Calif., June 8, 2020 /PRNewswire/ — Edwards Lifesciences Corporation (NYSE: EW), the global leader in patient-focused innovations for structural heart disease and critical care monitoring, today announced that it has received Chinese regulatory approval for the Edwards SAPIEN 3 transcatheter heart valve for the treatment of patients suffering from severe, symptomatic aortic […]
Edwards SAPIEN 3 TAVI Receives Expanded Approval In Europe
Superior TAVI Valve Indicated for All Patients Diagnosed with Aortic Stenosis IRVINE, Calif., Nov. 6, 2019 /PRNewswire/ — Edwards Lifesciences Corporation (NYSE: EW), the global leader in patient-focused innovations for structural heart disease and critical care monitoring, today announced that it has received CE Mark to expand use of the Edwards SAPIEN 3 […]
Edwards SAPIEN 3 TAVR Demonstrates Significant Health Status Improvements for Low-Risk Patients
SAN FRANCISCO, Sept. 29, 2019 /PRNewswire/ — Edwards Lifesciences Corporation (NYSE: EW), the global leader in patient-focused innovations for structural heart disease and critical care monitoring, today announced new data demonstrating early and sustained health status advantages for severe aortic stenosis (AS) patients at low surgical risk treated with the Edwards SAPIEN 3 valve. […]
Edwards SAPIEN 3 TAVR Receives FDA Approval For Low-Risk Patients
IRVINE, Calif., Aug. 16, 2019 /PRNewswire/ — Edwards Lifesciences Corporation (NYSE: EW), the global leader in patient-focused innovations for structural heart disease and critical care monitoring, today announced U.S. Food and Drug Administration (FDA) approval to expand use of the Edwards SAPIEN 3 and SAPIEN 3 Ultra transcatheter heart valve systems to the treatment […]