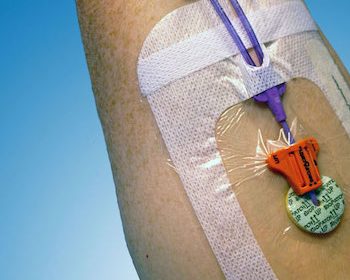
PLYMOUTH, Minn.–(BUSINESS WIRE)–Interrad Medical, a privately held medical device company, announces a new recommendation for use of SecurAcath on COVID-19 patients. The recommendation document was released on April 5, 2020 by a group of experts from the Long Term Central Venous Access Group (GAVeCeLT) and covers important aspects of vascular […]
Tag: SecurAcath
SecurAcath Selected for Fast-Track NHS Use Through New Innovation and Technology Payment Program
PLYMOUTH, Minn.–(BUSINESS WIRE)–Interrad Medical, a privately held medical device company, announces the SecurAcath Subcutaneous Catheter Securement Device has been selected for the Innovation and Technology Payment (ITP) Program by the National Health Service (NHS) England. The ITP Program is designed to remove significant barriers to the spread and adoption of […]
SecurAcath Receives NICE Recommendation: Highlights Massive Cost Savings from Improved Catheter Securement
PLYMOUTH, Minn.–(BUSINESS WIRE)–Interrad Medical, a privately held medical device company, announces the SecurAcath Subcutaneous Engineered Stabilization Device is now recommended by the National Institute for Health and Care Excellence (NICE). The SecurAcath underwent a detailed assessment under the Medical Technologies Evaluation Program. As a result, NICE has published guidance supporting […]