LOS ANGELES–(BUSINESS WIRE)–Sensydia, a medical technology company advancing non-invasive cardiac assessment, today announced the appointment of Rusty Page as President and Chief Operating Officer. Page joins Sensydia from publicly traded Neuronetics, Inc., where he served as SVP, Chief Information & Operations Officer. He brings more than two decades of senior […]
Tag: Sensydia
Sensydia Completes Fifth Study for Heart-Sound AI
LOS ANGELES–(BUSINESS WIRE)–Non-invasive cardiac assessment company Sensydia announced today that it has completed its 50-subject development study at the University of Minnesota (UMN). This study was conducted at UMN to collect data for its innovative AI-powered, non-invasive Cardiac Performance System (CPS™) that uses heart sound analysis to enable earlier detection and more effective therapy […]
Sensydia Secures $3M NIH Grant to Advance AI-Based Cardiac Assessment Platform
NIH STTR Fast-Track grant helps propel Sensydia’s non-invasive CPS platform building on recent funding round LOS ANGELES–(BUSINESS WIRE)–Non-invasive cardiac diagnostic company Sensydia announced today that it was awarded a Fast-Track Small Business grant from the National Heart, Lung, and Blood Institute (NHLBI), part of the National Institutes of Health (NIH). The award provides […]
Sensydia raises $8M round to advance breakthrough CPS cardiac assessment platform
Sensydia continues to push the boundaries of AI in heart function assessment LOS ANGELES–(BUSINESS WIRE)–Non-invasive cardiac assessment company Sensydia today announced it has raised approximately $8M in new funding from an expanded syndicate of investors. Proceeds of the financing will enable the company to prepare for commercialization of its groundbreaking non-invasive cardiac […]
Sensydia Achieves Study Target for Heart-Sound AI
— Completion of enrollment for this 225-subject study is a key milestone on the path to delivering the first device for non-invasive and accurate measurement of pulmonary pressure — LOS ANGELES–(BUSINESS WIRE)–Non-invasive cardiac assessment company Sensydia announced today that it has completed its 225-subject development study at the University of Pittsburgh Medical Center (UPMC). […]
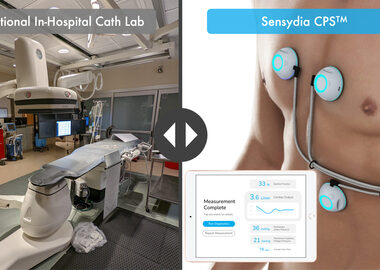
Sensydia Receives FDA Breakthrough Device Designation for CPSTM Non-Invasive Cardiac Monitoring Device
– External device uses heart sound and AI to deliver rapid cardiac function assessments via tablet to expand hemodynamic evaluation beyond the Cath lab – LOS ANGELES–(BUSINESS WIRE)–Sensydia, an innovator in rapid, non-invasive measurement of critical cardiac function, today announced that its Cardiac Performance System (CPSTM) has been granted Breakthrough Device Designation by the […]
Sensydia Appoints Anthony Arnold as President and Chief Executive Officer
LOS ANGELES–(BUSINESS WIRE)–Sensydia Corporation, an innovator in rapid, non-invasive measurement of critical cardiac function, announced that industry leader Anthony Arnold has been appointed as chief executive officer and president and joins the Board of Directors. Arnold is a pioneer in bioelectronic medicine and surgical navigation and brings more than 20 years of […]