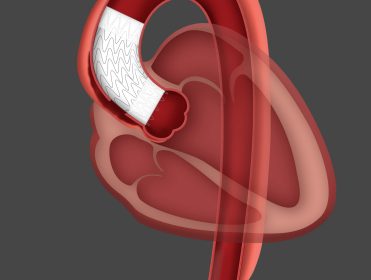
Houston, TX, May 27, 2020 (GLOBE NEWSWIRE) — via NEWMEDIAWIRE — AngioSoma, Inc. (OTC: SOAN) (“AngioSoma” or the “Company”) is pleased to announce that the Company is focusing on development and commercialization of our coating for metal stents. A stent is an expandable “scaffold-like” device, usually constructed of a metallic material, that […]
Tag: stent
Medtronic Launches Telescope(TM) Guide Extension Catheter to Support Complex Coronary Cases
DUBLIN – May 16, 2019 – Medtronic plc (NYSE:MDT), a global leader in percutaneous coronary intervention (PCI) innovation, today announced its entrance into the guide extension catheter market with the global launch of the Telescope(TM) Guide Extension Catheter, a newly designed catheter used to provide additional backup support and access to […]
Gore Announces Successful Patient Implant of Endovascular Stent Graft for the Ascending Aorta
FLAGSTAFF, Ariz.–(BUSINESS WIRE)–W. L. Gore & Associates, Inc. (Gore) today announced the first implant in conjunction with the Gore ARISE Study of the GORE® Ascending Stent Graft, an investigational device and the only endovascular stent graft specifically designed to treat Type A dissections of the ascending aorta. The successful procedure took place […]
Cook Medical Introduces a New Length of Zilver® PTX®
BLOOMINGTON, Ind.–(BUSINESS WIRE)–Cook Medical has introduced the 140 mm-length Zilver® PTX® Drug-Eluting Peripheral Stent in both 6 and 7 mm diameters in the U.S. The longer length comes after an expanded indication approval by FDA to treat total lesion lengths up to 300 mm per patient. In addition, the product […]
NaviGate Cardiac Structures Inc. (“NCSI”) reports “excellent valvular function” at 1-year follow-up of first patient to receive GATE™ tricuspid valved stent via transjugular access
LAKE FOREST, Calif.–(BUSINESS WIRE)–NaviGate Cardiac Structures Inc. (“NCSI”) announced today that the first patient to receive its replacement tricuspid valved stent via transjugular access has reached one-year post-procedure with excellent valvular function. This patient, along with 15 others treated to date under compassionate use protocols for severe tricuspid regurgitation, have received […]
CERENOVUS Receives FDA Clearance For Next Generation Stent Retriever Device Used To Treat Ischemic Stroke
IRVINE, Calif., May 21, 2018 /PRNewswire/ — CERENOVUS, part of the Johnson & Johnson Medical Devices Companies, announced today it has received 510(k) clearance from the U.S. Food and Drug Administration (FDA) for EMBOTRAP II Revascularization Device, a next generation stent retriever used to capture and remove life-threatening blood clots from […]
Abbott’s XIENCE Sierra™ Heart Stent Receives National Reimbursement in Japan to Treat People with Coronary Artery Disease
ABBOTT PARK, Ill., May 2, 2018 /PRNewswire/ — Abbott (NYSE: ABT) today announced that Japan’s Ministry of Health Labour and Welfare (MHLW) granted national reimbursement for XIENCE Sierra™, the newest generation of the company’s gold-standard XIENCE everolimus-eluting coronary stent. XIENCE Sierra improves upon previous versions of XIENCE with an enhanced stent design, a new delivery […]
FDA Approves Stryker’s Neuroform Atlas Stent System to Treat Wide Neck Aneurysms
KALAMAZOO, Michigan, USA, Nov. 9, 2017 /PRNewswire/ — Stryker Corporation announced today that the U.S. Food and Drug Administration has approved the Neuroform Atlas™ Stent System for marketing under a humanitarian device exemption (HDE). The device is to be utilized in conjunction with neurovascular embolic coils for the treatment of wide neck, […]
Abbott Shows off Landmark XIENCE Stent Data
DENVER, Oct. 30, 2017 /PRNewswire/ — Abbott (NYSE: ABT) today announced that patients who underwent minimally-invasive implantation with a XIENCE coronary stent for left-main coronary artery disease had the same long-lasting health outcomes at three years but felt better more quickly than patients who underwent open-heart surgery. The data were presented during […]
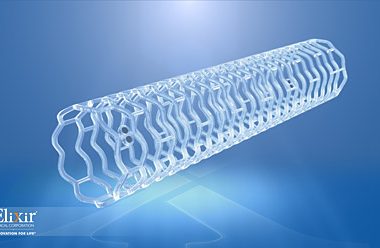
Elixir Medical Corporation to Unveil Game-Changing DynamXTM Metallic Stent at the 29th Transcatheter Cardiovascular Therapeutics Conference
MILPITAS, Calif.–(BUSINESS WIRE)–Elixir Medical Corporation, a leader in the development of breakthrough adaptive remodeling technologies designed to mimic the normal arterial function after cardiovascular and peripheral vascular disease intervention, announced today it will unveil a game-changing metallic drug eluting stent (DES) platform at this year’s Transcatheter Cardiovascular Therapeutics (TCT) conference […]