ALAMEDA, Calif. – January 17, 2018 – Penumbra, Inc. (NYSE: PEN), a global healthcare company focused on innovative therapies, today announced key events at the upcoming International Stroke Conference 2018 meeting (ISC 2018) to be held January 24-26 at the Los Angeles Convention Center. These events include results from two […]
Tag: stroke
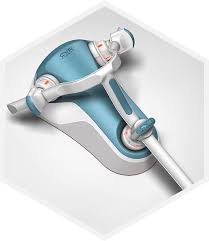
MEDTRONIC RECEIVES FDA CLEARANCE FOR RIPTIDE(TM) ASPIRATION SYSTEM
DUBLIN – January 16, 2018 – Medtronic plc (NYSE:MDT) today announced that the company’s Neurovascular business unit received U.S. Food and Drug Administration (FDA) clearance of the Riptide(TM) Aspiration System, adding a valuable tool to the Acute Ischemic Stroke (AIS) product portfolio. The Riptide Aspiration System is designed to retrieve thrombus […]
First Patient Enrolled in U.S. IDE Study To Evaluate the Potential of New Device To Reduce Stroke Risk in Atrial Fibrillation Patients
IRVINE, Calif., Jan. 11, 2018 /PRNewswire/ — Johnson & Johnson Medical Devices Companies* today announced that Biosense Webster, Inc., a worldwide leader in the diagnosis and treatment of heart arrhythmias, enrolled the first patient in the WaveCrest® Investigational Device Exemption (IDE) Trial. The study will evaluate the safety and effectiveness of the WaveCrest® Left Atrial […]
Vesalio Announces NeVa™ Stroke Treatment Product CE Mark Certification
Nashville, TN, October 31, 2017 –(PR.com)– Vesalio announces the CE Mark certification of its first product line, the proprietary NeVa™ neurothrombectomy platform for the treatment of strokes. Developed by a team of physicians that treat stroke, the NeVa mechanical thrombectomy technology was designed to improve “first pass” success, by effectively […]
Five-year Follow-up Data Demonstrate the WATCHMAN™ Left Atrial Appendage Closure Device Provides Stroke Risk Reduction Comparable to Warfarin Therapy
DENVER and MARLBOROUGH, Mass., Nov. 2, 2017 /PRNewswire/ — Boston Scientific (NYSE: BSX) announced final five-year outcomes data from the PREVAIL study of the WATCHMAN™ Left Atrial Appendage Closure (LAAC) Device today during a late-breaking clinical trial session at the 29th Transcatheter Cardiovascular Therapeutics (TCT), the annual scientific symposium of the Cardiovascular Research Foundation, in Denver. The […]
Bayer (BAY) Release: In Canadian-Led Phase III Clinical Study, Xarelto® When Combined With ASA Significantly Lowered The Combined Risk Of Stroke, Cardiovascular Death, And Heart Attack In Patients With Chronic Coronary Or Peripheral Artery Disease By 24%
Bleeding rates were low, and while major bleeding was increased, notably, there was no significant increase in intracranial or fatal bleeding1. This combination regimen demonstrated a substantial improvement in net clinical benefit of 20%1. Data from Canadian-led COMPASS study, revealed at ESC Congress 2017, included 27,395 patients globally and […]
Canon Virginia, Inc. Collaborates with CVR Medical to Manufacture Intuitive, Next-Generation Stroke Prevention Technology
NEWS PROVIDED BY Canon U.S.A., Inc. 10:18 ET NEWPORT NEWS, Va., Aug. 7, 2017 /PRNewswire/ — After recently extending its full range of services to include medical device contract manufacturing and receiving ISO 13485 certification, Canon Virginia, Inc. (CVI), a wholly owned subsidiary of Canon U.S.A., Inc., announces an intent to collaborate with CVR Global […]
Rapid Medical Snags $9 Million to Advance Commercialization of Stroke Products
YOKNEAM, Israel, July 17, 2017 /PRNewswire/ — Rapid Medical, a company focused on the development of neurovascular interventional devices, announced today that it has completed a Series B financing of $9M to advance the commercialization of the company’s minimally invasive stroke treatment and prevention products, the TIGERTRIEVER Revascularization Device, and the COMANECI, Adjustable Remodeling Mesh. […]
Sensome, Formerly Instent, Raises €4.7 Million To Bring The World’s First Connected Stroke Guidewire To Patients
PARIS–(BUSINESS WIRE)–Sensome, formerly Instent, a company developing a revolutionary micro-sensor technology for smart medical devices out of France’s Ecole polytechnique, announced today that it has completed its €4.7m seed round to bring its first product to clinical trials. Venture capital firm Kurma Diagnostics led a syndicate with the new Paris-Saclay […]
W. L. Gore & Associates Announces Positive Results From REDUCE Clinical Study For PFO Closure
FLAGSTAFF, Ariz.–(BUSINESS WIRE)–W. L. Gore & Associates, Inc. (Gore) announces positive results from its REDUCE Study assessing closure of patent foramen ovale (PFO) for the reduction of recurrent ischemic stroke and new brain infarct. The data were shared May 16 at the European Stroke Organisation Conference (ESOC) in Prague, Czech […]