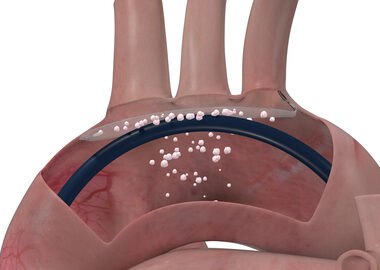
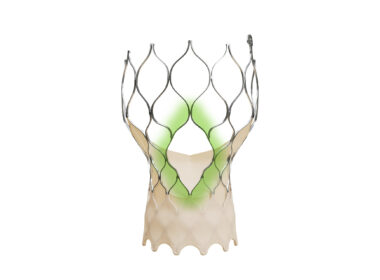
ATLANTA, March 28, 2025 /PRNewswire/ — MiRus is pleased to announce the launch of the US multi-center Early Feasibility Study of the Siegel™ 8-Fr aortic transcatheter heart valve (THV). The first two cases were successfully performed this week by Pradeep K. Yadav MD, Director of…
Tag: TAVR
German Medtech Protembis Gets €20 Million EIB Backing for Technology to Protect Brain During Heart Treatment
July 30, 2024 01:00 AM Eastern Daylight Time LUXEMBOURG & AACHEN, Germany–(BUSINESS WIRE)–The European Investment Bank (EIB) is providing €20 million in venture-debt financing to German medical-technology company Protembis to develop a next-generation device for protecting the brains of patients who undergo certain heart treatments. “This agreement demonstrates our commitment […]
Edwards Launches the SAPIEN 3 Ultra RESILIA Valve in Europe With Technology to Enhance Durability
NYON, Switzerland–(BUSINESS WIRE)–Edwards Lifesciences today announced the European launch of the SAPIEN 3 Ultra RESILIA valve, the only transcatheter aortic heart valve to incorporate the company’s breakthrough RESILIA tissue technology, designed to extend the valve’s durability.* Edwards’ SAPIEN 3 Ultra RESILIA valve recently received CE Mark† for use in patients with […]
Medtronic announces FDA approval of newest-generation Evolut TAVR system for treatment of symptomatic severe aortic stenosis
The Evolut™ FX+ TAVR system leverages market-leading valve performance with addition of larger windows to facilitate coronary access DUBLIN, March 27, 2024 /PRNewswire/ — Medtronic plc (NYSE: MDT), a global leader in healthcare technology, today announced that the United States Food and…
Large, Real-world Studies Demonstrate Continued Excellent Outcomes for Patients Receiving Edwards SAPIEN TAVR
WASHINGTON–(BUSINESS WIRE)–Edwards Lifesciences (NYSE: EW) announced today at Cardiovascular Research Technologies (CRT) 2024 the compelling results from two large, real-world studies based on TVT Registry data that demonstrated continued excellent outcomes for patients treated with the Edwards SAPIEN valve platform. A study of Edwards’ latest TAVR technology, the SAPIEN 3 Ultra RESILIA valve, found lower rates of paravalvular leak (PVL) at 30 days, lower echo-derived gradients and larger eff
Teleflex Announces First Patient Enrollment in ACCESS-MANTA™ Registry
Registry Intends to Examine Contemporary On-Label Use of the MANTA™ Vascular Closure Device Including Appropriate Patient Selection and Proper Vascular Access WAYNE, Pa., Nov. 16, 2023 (GLOBE NEWSWIRE) — Teleflex Incorporated (NYSE: TFX), a leading global provider of medical technologies, today announced the first patient enrollment in a clinical registry […]
Teleflex Receives FDA Clearance for the Wattson® Temporary Pacing Guidewire
Company expands Structural Heart Portfolio with the first commercially available bipolar temporary pacing guidewire designed specifically for use during transcatheter aortic valve replacement (TAVR) and balloon aortic valvuloplasty (BAV) WAYNE, Pa., June 07, 2023 (GLOBE NEWSWIRE) — Teleflex Incorporated (NYSE: TFX), a leading global provider of medical technologies, today announced […]
Valve Medical Announces Successful First-in-Human Implantation of Ultra-low Profile TAVR Valve in Israel
TEL AVIV, Israel, May 31, 2023 /PRNewswire/ — Valve Medical, a wholly owned subsidiary of Medinol Ltd. is pleased to announce today the successful First-in-Human (FIH) implantation of their advanced Transcatheter Aortic Valve Replacement (TAVR) system in Israel, introducing a revolutionary advancement in Structural Heart procedures with the world’s first modular valve. The […]
JenaValve Announces First Trilogy Heart Valve Systems Implanted in Asia
IRVINE, Calif., May 18, 2023 (GLOBE NEWSWIRE) — JenaValve Technology, Inc., developer and manufacturer of differentiated transcatheter aortic valve replacement (TAVR) systems, today announced the completion of the first two Trilogy Heart Valve System implantations in Asia. The two procedures were performed by Dr. Michael Lee and team at Queen […]
DATA FROM BENCHMARK REGISTRY DEMONSTRATE IMPROVED TAVR EFFICIENCY WITH PRESERVED PATIENT SAFETY
PARIS, May 17, 2023 /PRNewswire/ — Edwards Lifesciences (NYSE: EW) announced today that new data from the Benchmark Registry in Europe demonstrated the safety and effectiveness of this streamlined treatment pathway for patients receiving transcatheter aortic valve replacement (TAVR) with balloon-expandable valves. The data were presented in a late-breaking clinical data session at EuroPCR 2023. […]