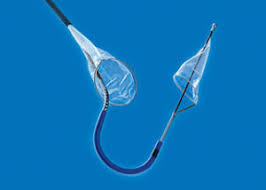
SANTA ROSA, Calif.–(BUSINESS WIRE)– Claret Medical, developer of the Sentinel® Cerebral Protection System, the first FDA-cleared cerebral protection device for transcatheter aortic valve replacement (TAVR), today announced that it has closed on a Series C financing of $14.5 million led by Lightstone Ventures, with participation from existing investors Easton Capital, HealthCor Partners, […]
Tag: TAVR
Study of Patients with Kidney Disease post TAVR
Study Evaluates Patients With Kidney Disease After Undergoing TAVR By Cardiac Interventions Today October 16, 2017—The American College of Cardiology (ACC) announced the publication of a study demonstrating a relatively low rate of patients with chronic kidney disease undergoing transcatheter aortic valve replacement (TAVR) who eventually need to start dialysis. […]
New Study Shows That Sentinel Cerebral Protection System Significantly Reduces Stroke and Mortality Associated with Transcatheter Aortic Valve Replacement (TAVR)
SANTA ROSA, Calif.–(BUSINESS WIRE)–Claret Medical® today announced publication of a new study in the Journal of the American College of Cardiology (JACC): Cardiovascular Interventions that underscores the role of the Sentinel Cerebral Protection System (CPS) in significantly reducing the early occurrence of stroke associated with transcatheter aortic valve replacement (TAVR). The study […]
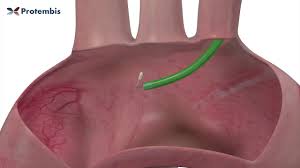
Protembis Announces Successful First-in-Human Use of Its ProtEmbo® Cerebral Protection System in European Trial
AACHEN, Germany–(BUSINESS WIRE)–Protembis GmbH, a privately held medical device company, announced today the first clinical applications of its ProtEmbo® Cerebral Protection System to complement a transcatheter aortic valve replacement (TAVR) procedure. The ProtEmbo® System is an intra-aortic filter device that deflects embolic material arising during TAVR away from the brain. Darren Mylotte, […]
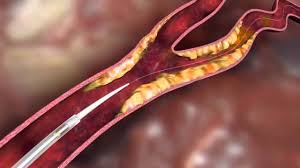
InspireMD’s Cguard Carotid Embolic Prevention System Featured At The SOLACI CACI Congress Of Cardiology 2017 In Buenos Aires
TEL AVIV, ISRAEL–(Marketwired – August 03, 2017) – InspireMD, Inc. (NYSE MKT: NSPR) (NYSE MKT: NSPR.WS) (“InspireMD” or the “Company”), a leader in embolic prevention systems (EPS) / thrombus management technologies and neurovascular devices, today announced that an endovascular interventional procedure featuring the CGuard™ EPS performed by the team of Dr. Anibal Damonte, Interventional […]
Medtronic Expands TAVR Access to More Patients With Symptomatic, Severe Aortic Stenosis Upon Intermediate Risk FDA Approval
Press Release DUBLIN – July 10, 2017 – Medtronic plc (NYSE: MDT) today announced the expanded U.S. Food and Drug Administration (FDA) approval of the self-expanding CoreValve(TM) Evolut(TM) transcatheter aortic valve replacement (TAVR) platform to include patients with symptomatic severe aortic stenosis who are at an intermediate risk for open-heart surgery. […]
Innovative Cardiovascular Solutions Raises $5.0 Million in Series B Financing for Advanced Device Development and Initiation of U.S. Clinical Activities
KALAMAZOO, Mich., July 11, 2017 /PRNewswire/ — Innovative Cardiovascular Solutions, LLC (ICS), a privately-held clinical stage company headquartered in Kalamazoo, MI, announced today completion of an oversubscribed Series B round of financing totaling $5M USD. The company is currently developing the EMBLOK™ Embolic Protection System which is designed for use in Transcatheter Aortic Valve Replacement […]
Medtronic Expands TAVR Access to More Patients With Symptomatic, Severe Aortic Stenosis Upon Intermediate Risk FDA Approval
DUBLIN – July 10, 2017 – Medtronic plc (NYSE: MDT) today announced the expanded U.S. Food and Drug Administration (FDA) approval of the self-expanding CoreValve(TM) Evolut(TM) transcatheter aortic valve replacement (TAVR) platform to include patients with symptomatic severe aortic stenosis who are at an intermediate risk for open-heart surgery. With hemodynamic […]
Edwards Lifesciences (EW)’ Novel Self-Expanding Transcatheter Heart Valve Demonstrates Excellent Early Patient Outcomes
PARIS, May 17, 2017 /PRNewswire/ — Edwards Lifesciences Corporation (NYSE: EW), the global leader in patient-focused innovations for structural heart disease and critical care monitoring, today announced late-breaking data at EuroPCR 2017 demonstrating excellent clinical outcomes for transcatheter aortic valve replacement (TAVR) patients treated with the Edwards CENTERA valve. These new […]
Venus Medtech’s TAVR Device Is Approved By CFDA, Creating A New Era Of Interventional Cardiology In China
HANGZHOU, China, April 28, 2017 /PRNewswire/ — Venus Medtech (Hangzhou) Inc., announced on April 25th that its transcatheter aortic valve system – Venus A-valve – has been approved by China Food and Drug Administration(“CFDA”) (registration no.: 20173460680) for sale in China. This marks the first-ever CFDA approved Transcatheter Aortic Valve Implantation […]