LithiX™ HC-IVL is a novel lithotripsy system designed to facilitate treatment of heart disease exacerbated by the presence of calcium within coronary artery plaques MY-IVL study enrolled 102 real world complex patients (130 lesions) with core lab adjudicated intravascular imaging analysis MILPITAS, Calif., Nov. 06, 2025 (GLOBE NEWSWIRE) — Elixir Medical, a developer of disruptive technologies to treat cardiovascular disease, announced results from the MY-IVL Study at the Transcatheter Cardiovascular Therapeutics (TCT) Conference 2025 in San Francisco. The data showed excellent clinical outcomes for LithiX™ Hertz Contact (HC) Intravascular Lithotripsy System (IVL) in the treatment of severely calcified coronary lesions among complex and high-risk percutaneous coronary intervention (PCI) patients. The MY-IVL Study evaluated 102 consecutive all-comer patients with notable severe calcified coronary artery disease treated with LithiX HC-IVL between April and September 2025 at Cardiac Vascular Sentral Kuala Lumpur Hospital (CVSKL), Malaysia’s first and leading private heart and vascular center. Angiographic and intravascular imaging from all patients was analyzed and adjudicated by an independent core lab MedStar Cardiovascular Research Network Core Laboratory at Washington Hospital Center, Washington, DC. The study included a broad range of clinically complex patients with multiple comorbidities clinically presenting with stable angina or acute coronary syndrome, including patients in cardiogenic shock, reflecting the device’s use and performance in everyday practice. Patient and lesion characteristics include: 102 patients with 130 lesions treatedAverage lesion length was 19.72 ± 14.09 mm and more than 30% of lesions were longer than 20 mm with an average length of 35.7 mm88% of lesions had severe calcificationCalcification in 41% of lesions was eccentric (arc of ≤ 270°), including 20% of lesions with an arc ≤ 180°94.6% of lesions were Class B2/C by American Heart Association (AHA) classification, which is associated with almost a two-fold risk of target lesion failure events within 30 days as compared to noncomplex lesions 1 Key clinical and procedural findings include: 96.1% of freedom from MACE at 30 days (Primary Safety Endpoint)Angiographic diameter stenosis of < 30% without intra-procedural MACE was achieved in 96.3% of patients following DES treatment There were no LithiX HC-IVL device-related angiographic procedural complications Key core lab adjudicated intravascular imaging results: Post procedure mean minimum stent area (MSA) was 6.89 mm², exceeding the powered performance goal (PG) of 4.9 mm² (p 100% was achieved in all lesion morphologies, including concentric and eccentric calcification “LithiX represents an important advancement in PCI. In our experience, its notable advantages include versatility and high treatment effect in modifying the most complex calcified lesions using a simpler procedure flow, particularly in this study with only a single device used per procedure, including multiple and long lesions,” said Dr. Tamil Selvan Muthusamy, Consultant Cardiologist at CVSKL and Co-Principal Investigator of the MY-IVL Study. The LithiX HC-IVL System is an advanced intravascular lithotripsy technology designed to optimize device expansion in calcified coronary artery blockages during a PCI procedure. Unlike energy-based systems dependent on an external energy generator, LithiX device is a highly deliverable mechanical lithotripsy device using a balloon with integrated low-profile metallic hemispheres to create focal pressure amplification based on Hertz Contact Stress theory. “Our study demonstrates excellent calcium modification potential of this novel Hertz Contact Lithotripsy technology, with MSA above 100% in all treated lesions, even those with less than 180% degree arc of calcium, which, based on our experience, are often difficult to treat with existing calcium modification devices,” added Dr. Rosli Mohd Ali, Consultant Cardiologist at CVSKL and Co-Principal Investigator of the MY-IVL Study. The Hertz Contact Lithotripsy hemispheres work selectively on calcium within the lesion, breaking and fragmenting the hardened plaque under low balloon inflation pressure. The hemispheres (27 to 45 depending on device size) are designed to create highly localized points of amplified force to create deep and wide fractures at the multiple points of contact with calcium while being atraumatic to the adjacent non-calcified vessel due to the tissue’s elasticity. About Elixir Medical Elixir Medical Corporation, a privately held company based in Milpitas, California, develops disruptive platforms to treat coronary and peripheral artery disease. Our transformative technologies have multiple applications across the cardiovascular space capable of delivering improved clinical outcomes for millions of patients. Elixir Medical was named to Fast Company’s prestigious list of the World’s Most Innovative Companies of 2025 and Fierce Medtech’s 2025 Fierce 15 list. Visit us at www.elixirmedical.com and on LinkedIn and X. The LithiX HC-IVL System is CE Mark approved and is not available in the United States. Media Contact Richard Laermer, RLM PR, elixir@rlmpr.com (212) 741-5106 X 216 1 Konigstein M et al. J Am Heart Assoc. 2022;11:e025275. DOI: 10.1161/JAHA.121.025275
Tag: TCT
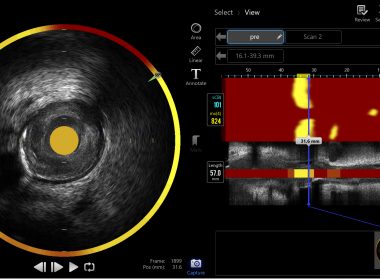
Conavi Medical to Highlight Novasight Hybrid™ Imaging at TCT 2025
Company to Participate in Educational Imaging Sessions and Feature Hybrid IVUS/OCT Imaging TechnologyTORONTO, Oct. 23, 2025 (GLOBE NEWSWIRE) — Conavi Medical Inc. (TSXV: CNVI) (OTCQB: CNVIF), a commercial-stage medical device company focused on intravascular imaging solutions, today announced that it will participate in the upcoming Transcatheter Cardiovascular Therapeutics (TCT) 2025 conference, taking place October 25 – 28, 2025, at the Moscone Center in San Francisco, California. Attendees will have the opportunity to learn more about Conavi’s hybrid intravascular imaging technology, which combines intravascular ultrasound (IVUS) and optical coherence tomography (OCT) to provide complementary insights into coronary anatomy. Conavi will share clinical images acquired using its first-generation Novasight Hybrid™ imaging system and provide information on the potential role of hybrid IVUS/OCT imaging in guiding complex percutaneous coronary interventions (PCI). “TCT provides an important forum for clinical education and scientific exchange in interventional cardiology,” said Thomas Looby, President and CEO of Conavi Medical. “We look forward to engaging with physicians and thought leaders to discuss how hybrid intravascular imaging can enhance procedural decision-making and improve patient outcomes.” The next-generation version of Conavi’s Novasight Hybrid system was submitted to the U.S. Food and Drug Administration (FDA) for 510(k) clearance in September 2025. Conavi’s first-generation version previously received FDA 510(k) clearance and Health Canada approval and was used at seven luminary cardiovascular care hospitals in North America. Intravascular Imaging Education SessionsConavi will also participate in the Intravascular Imaging & Training sessions at the Imaging & Training Center, held throughout the day on Sunday, October 26, and Monday, October 27. These physician-led sessions will feature expert case presentations illustrating how intravascular imaging can be used to guide challenging PCI procedures, including those involving calcified lesions, acute coronary syndrome (ACS), and in-stent restenosis (ISR). Conference Details Event: Transcatheter Cardiovascular Therapeutics (TCT) 2025Dates: October 26 – 29, 2025Location: Moscone Center, San Francisco, CaliforniaConavi Medical Booth: #2126 About TCT The Transcatheter Cardiovascular Therapeutics (TCT) conference, organized by the Cardiovascular Research Foundation (CRF), is the world’s leading educational meeting specializing in interventional cardiovascular medicine. TCT brings together thousands of physicians, scientists, and industry leaders from around the globe to present the latest research, live cases, and innovations in device-based therapies for coronary, structural, and endovascular disease. About Conavi Medical Conavi Medical is focused on designing, manufacturing, and marketing imaging technologies to guide common minimally invasive cardiovascular procedures. Its patented Novasight Hybrid™ System is the first to combine intravascular ultrasound (IVUS) and optical coherence tomography (OCT) into a single device, enabling simultaneous and co-registered imaging of coronary arteries. The first-generation Novasight Hybrid™ System received regulatory clearance in the U.S., Canada, China, and Japan. For more information, visit conavi.com. Cautionary Statement Regarding Forward-Looking Information This news release contains “forward-looking statements” within the meaning of applicable Canadian and U.S. securities laws, which reflect the current expectations of management of Conavi’s future growth, results of operations, performance and business prospects and opportunities. Forward-looking statements are frequently, but not always, identified by words such as “may”, “would”, “could”, “will”, “anticipate”, “believe”, “plan”, “expect”, “intend”, “estimate”, “potential for” and similar expressions, although these words may not be present in all forward-looking statements. Forward-looking statements that appear in this release may include, without limitation, references to Conavi’s plans for the commercialization of its Novasight Hybrid™ System and expected FDA clearance and the commercial launch of next generation Novasight in the U.S. These forward-looking statements reflect management’s current beliefs with respect to future events, and are based on information currently available to management that, while considered reasonable by management as of the date on which the statements are made, are inherently subject to significant business, economic and competitive uncertainties and contingencies which could result in actions, events, conditions, results, performance or achievements to be materially different from those projected in the forward-looking statements. Forward-looking statements involve significant risks, uncertainties and assumptions and many factors could cause Conavi’s actual results, performance or achievements to be materially different from any future results, performance or achievements that may be expressed or implied by such forward-looking statements. Such factors and assumptions include, but are not limited to, Conavi’s ability to retain key personnel; its ability to execute on its business plans and strategies; and other factors listed in the “Risk Factors” sections of the joint information circular of Conavi dated August 30, 2024 and in the final short form prospectus of Conavi dated April 15, 2025 (each of which may be viewed at www.sedarplus.com). Should one or more of these risks or uncertainties materialize, or should assumptions underlying the forward-looking statements prove incorrect, actual results, performance, or achievements may vary materially from those expressed or implied by the forward-looking statements contained in this news release. These factors should be considered carefully, and prospective investors should not place undue reliance on the forward-looking statements. Although the forward-looking statements contained in the news release are based upon what management currently believes to be reasonable assumptions and Conavi has attempted to identify important factors that could cause actual actions, events, conditions, results, performance or achievements to differ materially from those described in forward-looking statements, Conavi cannot assure prospective investors that actual results, performance or achievements will be consistent with these forward-looking statements. Except as required by law, Conavi expressly disclaims any intention or obligation to update or revise any forward-looking statements whether as a result of new information, future events or otherwise. Accordingly, investors should not place undue reliance on forward-looking statements. All the forward-looking statements are expressly qualified by the foregoing cautionary statements. Neither the TSX Venture Exchange nor its Regulation Services Provider (as that term is defined in the policies of the TSX Venture Exchange) accepts responsibility for the adequacy or accuracy of this press release. Contact:Stefano PiconeChief Financial Officer(416) 483-0100
Vesalio Announces Clinical Study Initiative for the Recently Launched pVasc Thrombectomy System and Planned Attendance at TCT, VIVA, and VEITH Meetings
Vesalio Introduces pVasc™ U.S. clinical study initiative to capture real-world data and optimize results for patients suffering from peripheral vascular disease PLANO, Texas, Oct. 25, 2024 /PRNewswire/ — Vesalio, a global thrombectomy company dedicated to advancing patient care across…
TCT 2021: Philips announces new innovations and clinical data supporting the treatment of patients with cardiovascular disease
November 2, 2021 Philips debuts three new solutions in the North American market that will help drive procedural innovation and workflow efficiency, adding to the company’s ecosystem of interventional imaging systems, and diagnostic and therapeutic devices New clinical data presented at TCT 2021: five-year outcomes of iFR-SWEDEHEART trial and new large-scale […]
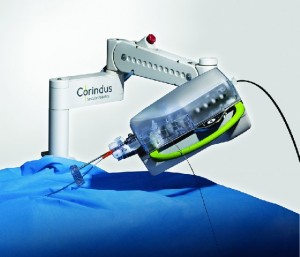
Corindus Technology Successfully used in Multiple Live Complex Robotic-Assisted Coronary Interventions at Transcatheter Cardiovascular Therapeutics 2018
WALTHAM, Mass.–(BUSINESS WIRE)–Corindus Vascular Robotics, Inc. (NYSE American: CVRS), a leading developer of precision vascular robotics, announced today that its CorPath GRX System was used for multiple complex robotic-assisted percutaneous coronary interventions (PCI) at the recent Transcatheter Cardiovascular Therapeutics (TCT) 2018 Conference. In the first case at University of Washington […]
Orchestra BioMed™ Announces Presentation of Positive 2-Year Clinical Results and Program Update for BackBeat® Cardiac Neuromodulation Therapy at TCT 2018
SAN DIEGO, Sept. 25, 2018 (GLOBE NEWSWIRE) — Orchestra BioMed, Inc. (“Orchestra BioMed” or the “Company”), a biomedical innovation company developing transformational therapeutic devices targeting major medical conditions, presented positive 2-year clinical results from its multicenter clinical trial for BackBeat® Cardiac Neuromodulation Therapy (CNT) at the Transcatheter Cardiovascular Therapeutics (TCT) 2018 in San […]
Landmark Lipid-Rich Plaque (LRP) Study Results Demonstrate the Ability to Accurately Identify Patients and Plaques at Risk for Major Coronary Events
BURLINGTON, Mass.–(BUSINESS WIRE)–Infraredx, Inc., a pioneer in intravascular imaging for mapping coronary artery disease, presented late-breaking clinical trial results for its landmark Lipid-Rich Plaque (LRP) Study today at the 30th Transcatheter Cardiovascular Therapeutics (TCT), the annual scientific symposium of the Cardiovascular Research Foundation. Cardiovascular disease remains the number one cause […]
Amaranth Presented MAGNITUDE (98 micron) Nine-Month Follow-up Clinical Results and Introduced DEFIANCE (85 micron) BRS Development Plan at TCT
MOUNTAIN VIEW, CA , Sept. 24, 2018 (GLOBE NEWSWIRE) — Amaranth Medical, a medical device company developing next-generation bioresorbable scaffolds, provided an update on the company’s sirolimus-eluting bioresorbable scaffold (BRS) products at the annual Transcatheter Cardiovascular Therapeutics (TCT) meeting during the following sessions: Didactic Session: Drug-Eluting Stents, Bioresorbable Scaffolds, and Coronary […]
Data Presented at TCT 2018 Shows Use of Impella and Best Practices Increases Cardiogenic Shock Survival
SAN DIEGO, Sept. 24, 2018 (GLOBE NEWSWIRE) — A new analysis of data from Abiomed’s (NASDAQ:ABMD) Impella® Quality (IQ) Database shows a relative increase of 24% in mean survival in acute myocardial infarction (AMI) cardiogenic shock patients since Impella’s cardiogenic shock FDA post-market approval. Part of the reason for the increase was a […]
Corindus Announces First Live Transmission of Remote Robotic Demonstration Performed at Transcatheter Cardiovascular Therapeutics 2018 Conference
WALTHAM, Mass.–(BUSINESS WIRE)–Corindus Vascular Robotics, Inc. [NYSE American: CVRS], a leading developer of precision vascular robotics, announced the first live transmission of a remote interventional procedure using the company’s CorPath® platform was performed at the Transcatheter Cardiovascular Therapeutics (TCT) Conference in San Diego, CA on September 22, 2018. The remote interventional […]