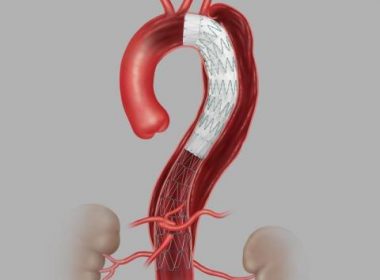
Global “DISSECT-N” Post-Market Study to Broaden Evidence Base for Safety and Effectiveness of Commercially Available Endovascular Repair Technology DUBLIN, July 06, 2020 (GLOBE NEWSWIRE) — Medtronic plc (NYSE:MDT), the global leader in medical technology, today announced the start of a prospective, observational, global, multi-center, real-world, post-market study to evaluate the safety and effectiveness of […]
Tag: TEVAR
First US Patient Treated with Cook Medical’s Recently Approved Zenith® Dissection Endovascular System
BLOOMINGTON, Ind.–(BUSINESS WIRE)–Following the recent FDA approval of Cook Medical’s Zenith Dissection Endovascular System, Cooper University Health Care in Camden, New Jersey, has treated the first patient in the US with the device as part of Cook’s US commercial launch. “We are committed to helping patients by developing a variety […]
Gore Announces First Commercial In-Human Use of GORE® TAG® Conformable Thoracic Stent Graft with ACTIVE CONTROL System for TEVAR in Australia
FLAGSTAFF, Ariz.–(BUSINESS WIRE)–W. L. Gore & Associates, Inc. (Gore) today announced the first patient implant of the GORE® TAG® Conformable Thoracic Stent Graft with ACTIVE CONTROL System after being included on the Australian Register of Therapeutic Goods last month in Australia. The first implants were performed by Professor Ian Spark […]