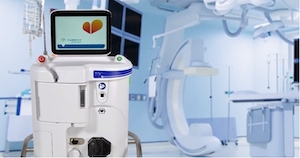
CHELMSFORD, Mass.–(BUSINESS WIRE)–ZOLL, an Asahi Kasei company, has been selected to exhibit SuperSaturated Oxygen (SSO2) Therapy, which delivers high levels of dissolved oxygen directly to the heart following severe heart attack, at the Vizient Innovative Technology Exchange. Vizient, Inc, the nation’s largest member-driven health care performance improvement company, will hold the […]
Tag: TherOx
First Patient Enrolled in AMIHOT III Trial Investigating ZOLL’s Supersaturated Oxygen Therapy in Heart Attack Patients
Study Evaluates the Effectiveness of TherOx Supersaturated Oxygen (SSO2) Therapy in Patients Presenting with Acute Anterior Myocardial Infarction with Successful Reperfusion Compared to Standard Therapy CHELMSFORD, Mass.–(BUSINESS WIRE)–ZOLL® Medical Corporation, an Asahi Kasei company that manufactures medical devices and related software solutions, announced today that the first patient has enrolled in […]
American Medical Association Issues New Category III CPT Code for ZOLL Medical’s TherOx SSO2 Therapy
CHELMSFORD, Mass.–(BUSINESS WIRE)–ZOLL® Medical Corporation, an Asahi Kasei company that manufactures medical devices and related software solutions, announced today that the American Medical Association (AMA) CPT Editorial Panel issued a new Category III CPT® code, effective July 1, 2021, for its TherOx® SuperSaturated Oxygen (SSO2) Therapy. The code, 0659T (transcatheter intracoronary infusion of […]
ZOLL Medical Receives FDA IDE Approval for Initiation of ISO Shock (Incorporating Supersaturated Oxygen in Shock) Feasibility Study
Study Evaluates the Safety and Feasibility of TherOx Supersaturated Oxygen (SSO2) Therapy in STEMI Patients presenting with Cardiogenic Shock CHELMSFORD, Mass.–(BUSINESS WIRE)–ZOLL® Medical Corporation, an Asahi Kasei company that manufactures medical devices and related software solutions, announced today the U.S. Food and Drug Administration (FDA) has approved an investigational device […]
ZOLL THEROX RECEIVES CE MARK APPROVAL FOR SUPERSATURATED OXYGEN THERAPY
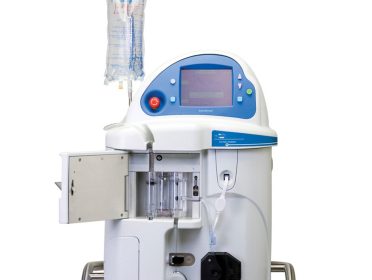
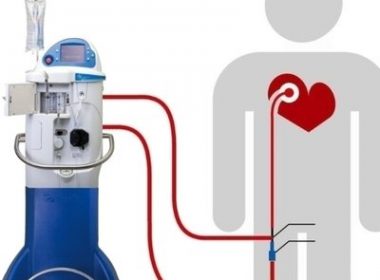
May 4, 2020 — CHELMSFORD, MASS. — ZOLL® Medical Corporation, an Asahi Kasei Group Company that manufactures medical devices and related software solutions, announced today it has received CE Mark approval to market and distribute its SuperSaturated Oxygen (SSO2) Therapy System in Europe. SSO2 Therapy provides interventional cardiologists with the first and only clinically proven […]
FDA Approves Next-Generation ZOLL® TherOx System for Widowmaker Heart Attack Patients
First and Only Treatment to Reduce Heart Muscle Damage Following Angioplasty and Stenting CHELMSFORD, Mass.–(BUSINESS WIRE)–ZOLL® Medical Corporation, an Asahi Kasei Group company that manufactures medical devices and related software solutions, announced today it has received U.S. Food and Drug Administration (FDA) approval of the second-generation TherOx System, which provides SuperSaturated […]
Zoll Medical Corporation Acquires TherOx, Inc.
CHELMSFORD, Mass.–(BUSINESS WIRE)–ZOLL® Medical Corporation, an Asahi Kasei Group Company that manufactures medical devices and related software solutions, today announced it has acquired TherOx, Inc., of Irvine, Calif., a privately held company. TherOx is focused on improving treatment of acute myocardial infarction (AMI) and markets systems to deliver SuperSaturated Oxygen (SSO2) […]
TherOx Receives FDA Approval for First Heart Attack Treatment Since PCI to Reduce Infarct Size
IRVINE, Calif.–(BUSINESS WIRE)–TherOx, Inc., a privately held medical device company focused on improving treatment of acute myocardial infarction (AMI), announced that the U.S. Food and Drug Administration (FDA) granted premarket approval for its SuperSaturated Oxygen (SSO2) Therapy. SSO2 Therapy provides interventional cardiologists with the first and only FDA-approved treatment beyond percutaneous […]
THEROX COMPLETES FINANCING AS IT PREPARES FOR AMI THERAPY SYSTEM LAUNCH
Supports U.S. Market Introduction of SuperSaturated Oxygen Therapy for Improved AMI Patient Outcomes Irvine, Calif., May 7, 2018 – TherOx®, a privately held medical device company focused on improving treatment of acute myocardial infarction (AMI), today announced completion of a debt financing for an undisclosed amount led by existing investor […]
TherOx Announces Its PMA Application for AMI Therapy System is Accepted for Filing by FDA
IRVINE, Calif.–(BUSINESS WIRE)–TherOx, Inc., a privately held medical device company focused on improving treatment of Acute Myocardial Infarction (AMI), announced that the U.S. Food and Drug Administration (FDA) has accepted the Premarket Approval (PMA) application for its Supersaturated Oxygen (SSO2) Therapy system. The second-generation SSO2 Therapy system is designed to reduce […]