PARK CITY, Utah, Nov. 30, 2023 /PRNewswire/ — Transit Scientific, a Utah-based medical device company specializing in innovative solutions for vascular procedures, has announced the FDA clearance of its microcatheter designed for the precise delivery of a broad range of embolic agents….
Tag: Transit Scientific
FDA Clears Angioplasty Platform to Crack, Break, and Dilate Stenosis in Calcified Cardiovascular Disease and Hemodialysis Fistula
Transformative XO Angioplasty Platform Expands with Low-Profile 2.2F and 3.8F Rapid-Exchange (RX) with the Ability to Treat Up to 20cm Lesions with (1) Inflation SALT LAKE CITY, Oct. 27, 2022 /PRNewswire/ — Transit Scientific, a pioneer in developing medical devices to treat calcified cardiovascular disease, dilate stenosed intimal hyperplasia, and access, cross, […]
Non-tapered Catheter Success in Critical Limb Ischemia Pedal Artery Access
XO Cross Catheter Platform successfully used in peripheral vascular procedures utilizing pedal artery retrograde access to treat complex Critical Limb Ischemia (CLI) patients. PARK CITY, Utah, Aug. 1, 2022 /PRNewswire/ — Transit Scientific reports multiple successful peripheral vascular procedures with the XO Cross Catheter Platform utilizing pedal retrograde access. The non-tapered 2Fr […]
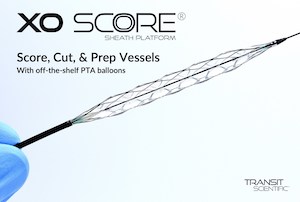
Continued Clinical Success Reported with Unique Cutting-Edge Vessel Prep Device
First-of-its-kind metal-alloy scoring, cutting, and constraining structure (CS) device continues to elevate angioplasty treatment of chronic dialysis fistulas and grafts. PARK CITY, Utah, June 30, 2022 /PRNewswire/ — Transit Scientific shares successful outcomes on multiple fistula and graft cases using its XO Score Sheath Platform along with off-the-shelf percutaneous transluminal angioplasty (PTA) balloons. […]
FDA Clears Four New Coronary Micro Support Catheters
Non-tapered metal-alloy support catheter platform receives FDA clearance for use in coronary and peripheral vasculature. Transit Scientific now has one of the largest platforms of FDA cleared microcatheters in the industry. PARK CITY, Utah, April 12, 2022 /PRNewswire/ — Transit Scientific announced the FDA clearance of its XO Cross® Support Catheter Platform to […]
New Scoring Sheath Delivers Low Pressure Angioplasty Treatment in Calcified Arteries
First-of-its kind metal-alloy angioplasty scoring sheath effective in treatment of critical arterial stenosis PARK CITY, Utah, Feb. 17, 2022 /PRNewswire/ — Transit Scientific announced continued success with its XO Score Sheath Platform to treat highly-stenosed cardiovascular lesions using low pressure peripheral transluminal angioplasty (PTA). “Calcified stenotic lesions in the iliac and common femoral […]
New Peripheral Vascular Microcatheters Save Procedure Time and Advance Treatment Capabilities
2F XO Cross 14 Microcath platform successfully used in challenging peripheral vascular procedures. PARK CITY, Utah, Jan. 11, 2022 /PRNewswire/ — Transit Scientific announced continued multicenter success with the XO Cross® platform. Dr. Jihad Mustapha, interventional cardiologist at Advanced Cardiac & Vascular Center, Grand Rapids, Michigan USA performed procedures using 2F XO Cross 14 Microcaths […]
FDA Clears 12 New XO Cross Microcatheters
12 new hydrophilic-coated micro and support catheters cleared by FDA for broad use in the peripheral vasculature. PARK CITY, Utah, Nov. 2, 2021 /PRNewswire/ — Transit Scientific announced the FDA clearance of new hydrophilic-coated XO Cross Microcatheters for guidewire support, exchange, and contrast media injection in the peripheral vasculature. “The XO Cross devices […]
Cutting-Edge Scoring Sheath Platform Earns CE Mark Approval
New metal-alloy scoring sheath CE Mark cleared for treatment of peripheral artery disease and end stage renal disease. PARK CITY, Utah, May 10, 2021 /PRNewswire/ — Transit Scientific announced it received CE Mark clearance in the European Union for the XO Score® Scoring Sheath Platform to facilitate dilation of stenotic material in the peripheral vasculature […]
Next-Generation Microcatheter Platform Earns CE Mark Approval
Novel XO Cross catheter platform CE Mark cleared for use in peripheral vasculature. PARK CITY, Utah, March 17, 2021 /PRNewswire/ — Transit Scientific announced it received CE Mark approval in the European Union for its non-tapered metal alloy XO CrossÒ Microcatheter platform. Microcatheters are commonly used to provide guidewire support, facilitate guidewire exchanges, access distal […]