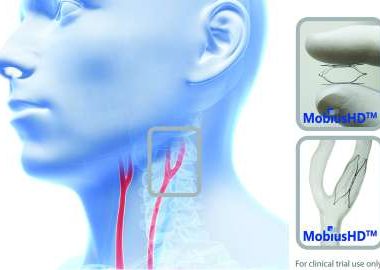
MOUNTAIN VIEW, Calif., Aug. 15, 2017 (GLOBE NEWSWIRE) — Vascular Dynamics, Inc., (VDI) a privately held medical device company developing novel solutions for the treatment of hypertension, today announces that the United States Food and Drug Administration (FDA) has approved the company’s Investigational Device Exemption (IDE) application to initiate its […]