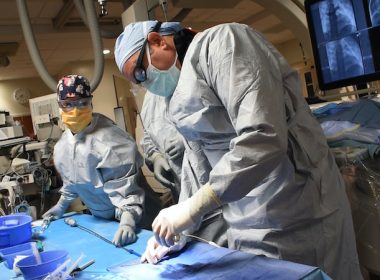
MT. OLIVE, N.J. & CARLSBAD, Calif.–(BUSINESS WIRE)–Catheter Precision, Inc., a medical device and technology company focused on cardiac electrophysiology, announces that more than 800 procedures have been performed in a number of leading U.S. and European hospitals utilizing the VIVO System that enables physicians to noninvasively identify an area of […]
Tag: VIVO
Catheter Precision’s VIVO™ Systems Used in More Than 20 Procedures at NYU Langone Hospital
MT. OLIVE, N.J. & CARLSBAD, Calif.–(BUSINESS WIRE)–Catheter Precision, Inc., a medical device and technology company focused on cardiac electrophysiology, announces the completion of more than 20 procedures at NYU Langone Hospital utilizing the VIVO System that enables physicians to identify the origin of arrhythmias pre-procedure. On September 12, 2022 privately […]
Catheter Precision Expands U.S. Customer Base for VIVO™ for Non-invasive Arrythmia Location Detection Before Ventricular Ablation Procedures
MT. OLIVE, N.J. & CARLSBAD, Calif.–(BUSINESS WIRE)–Catheter Precision, Inc. (“Catheter Precision”), a medical device company focused on cardiac electrophysiology, announces receipt of three commercial orders in the past 60 days as usage of the device continues to increase in the U.S. On September 12, 2022 privately held Catheter Precision announced […]
Catheter Precision Files a Method-of-Use U.S. Patent Application for its VIVO™ Technology
MT. OLIVE, N.J. & CARLSBAD, Calif.–(BUSINESS WIRE)–Catheter Precision, Inc. (“Catheter Precision”), a medical device company focused on cardiac electrophysiology, announces the filing of a new U.S. patent application for its VIVO™ (View Into Ventricular Onset) technology, as it continues its focus on patent protection for VIVO. On September 12, 2022 privately […]
Catheter Precision’s VIVO™ Technology to be Featured in Three Presentations at the Global Society of Cardiac Robotic Navigation Annual Meeting
MT. OLIVE, N.J. & CARLSBAD, Calif.–(BUSINESS WIRE)–Catheter Precision, Inc., a medical device and technology company focused on cardiac electrophysiology, announces that its lead product, VIVO™ (View Into Ventricular Onset), will be featured in presentations by three physicians working in the field of cardiac electrophysiology at the Sixth Annual Society of […]
VIVO™ System Cleared by FDA
MT. OLIVE, N.J., June 26, 2019 /PRNewswire/ — Catheter Precision, Inc., announced today that the U.S. Food and Drug Administration (FDA) has cleared its new VIVO™ (View into Ventricular Onset) system for market release in the United States. VIVO is a pre-procedure planning tool that offers 3D cardiac mapping to aid in localizing […]