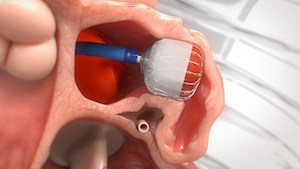
WATCHMAN FLX now the only LAAC technology in the United States that allows for either DAPT or OAC immediately following implantation MARLBOROUGH, Mass., Sept. 6, 2022 /PRNewswire/ — Boston Scientific Corporation ( NYSE: BSX) has received U.S. Food and Drug Administration (FDA) approval to expand the instructions for use labeling for the current-generation WATCHMAN FLX™ Left […]
Tag: Watchman FLX
Late-Breaking Study Results Reinforce Positive Real-World Outcomes with the WATCHMAN FLX™ LAAC Device
MARLBOROUGH, Mass., Feb. 28, 2022 /PRNewswire/ — Boston Scientific Corporation (NYSE: BSX) today announced positive results from a new analysis assessing real-world outcomes with the WATCHMAN FLX™ Left Atrial Appendage Closure (LAAC) Device. Presented during a late-breaking trial session at the Cardiovascular Research Technologies (CRT) 2022 meeting, the SURPASS analysis included data from more […]
Late-Breaking Trial Data at TVT Demonstrate Sustained Safety and Performance of WATCHMAN FLX™ Left Atrial Appendage Closure Device
PINNACLE FLX study meets 24-month endpoint, reinforces positive 12-month outcomes with next-generation device for patients with non-valvular atrial fibrillation MARLBOROUGH, Mass., July 21, 2021 /PRNewswire/ — Today, Boston Scientific (NYSE: BSX) announced positive 24-month results from the PINNACLE FLX clinical trial assessing the safety and efficacy of the next-generation WATCHMAN FLX™ Left Atrial […]
Extended FEops HEARTguide™ Offering Now Available
GENT, Belgium–(BUSINESS WIRE)–FEops, a digital health leader in personalized predictive planning for structural heart interventions, is proud to announce the new release of FEops HEARTguideTM. This includes the full range of the Boston Scientific WATCHMAN FLX™ devices, enhanced visualization for the prediction of paravalvular leak (PVL) and conduction abnormalities (CA) for […]
Boston Scientific Initiates Trial to Evaluate WATCHMAN FLX™ Left Atrial Appendage Closure Device as First-Line Treatment for People at Risk of Stroke
Trial to study next-generation device as treatment alternative to NOACs for broader population of patients with non-valvular atrial fibrillation MARLBOROUGH, Mass., Oct. 29, 2020 /PRNewswire/ — Boston Scientific Corporation (NYSE: BSX) has initiated the CHAMPION-AF clinical trial to evaluate the safety and efficacy of the WATCHMAN FLX™ Left Atrial Appendage Closure (LAAC) Device […]
PIH Health Good Samaritan Hospital Offers an Alternative to Long-Term Warfarin Medication for Atrial Fibrillation with Next-Generation Watchman FLX™ Implant
LOS ANGELES, Aug. 10, 2020 /PRNewswire/ — PIH Health Good Samaritan Hospital is one of the first hospitals in Los Angeles to offer the next-generation Watchman FLX™ Left Atrial Appendage Closure (LAAC) implant to patients with non-valvular atrial fibrillation (AF). The implant is an alternative to long-term warfarin medication. Up to 6 million Americans are […]
First hospitals in U.S. implant next-generation cardiac device to reduce risk of stroke
Texas Cardiac Arrhythmia Institute at St. David’s Medical Center, Los Robles Health System perform near simultaneous implantation of WATCHMAN FLX™ Left Atrial Appendage Closure (LAAC) AUSTIN, Texas, Aug. 7, 2020 /PRNewswire/ — On Aug. 5, 2020, physicians at the Texas Cardiac Arrhythmia Institute (TCAI) at St. David’s Medical Center in Austin, Texas, and Los Robles Health System in Thousand Oaks, California, became […]
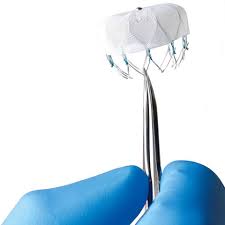
Boston Scientific Receives FDA Approval for Next-Generation WATCHMAN FLX™ Left Atrial Appendage Closure Device
New stroke risk reduction technology designed to advance procedural performance and safety, treat wider range of patients with non-valvular atrial fibrillation MARLBOROUGH, Mass., July 21, 2020 /PRNewswire/ — Boston Scientific Corporation (NYSE: BSX) announced it has received U.S. Food and Drug Administration (FDA) approval for the WATCHMAN FLX™ Left Atrial Appendage Closure (LAAC) Device. Experience […]
Boston Scientific Initiates Trial Comparing Left Atrial Appendage Closure to Direct Oral Anticoagulants for Stroke Risk Reduction Post-AFib Ablation
MARLBOROUGH, Mass., May 22, 2019 /PRNewswire/ — Boston Scientific Corporation (NYSE: BSX) has initiated the OPTION trial to compare safety and effectiveness of the next-generation WATCHMAN FLX™ left atrial appendage closure (LAAC) platform to first-line oral anticoagulants (OAC) – including direct oral anticoagulants (DOAC) and warfarin – for stroke risk reduction in patients with […]
Boston Scientific Receives CE Mark for Next Generation WATCHMAN FLX™ Left Atrial Appendage Closure Device
MARLBOROUGH, Mass., March 13, 2019 /PRNewswire/ — Boston Scientific Corporation (NYSE: BSX) announced it has received CE Mark and initiated a limited market release of the next generation WATCHMAN FLX™ Left Atrial Appendage Closure (LAAC) Device in Europe. Patients with AF are five times more likely to suffer a stroke than someone with a normal heart […]